Contents
What are oncogenes?
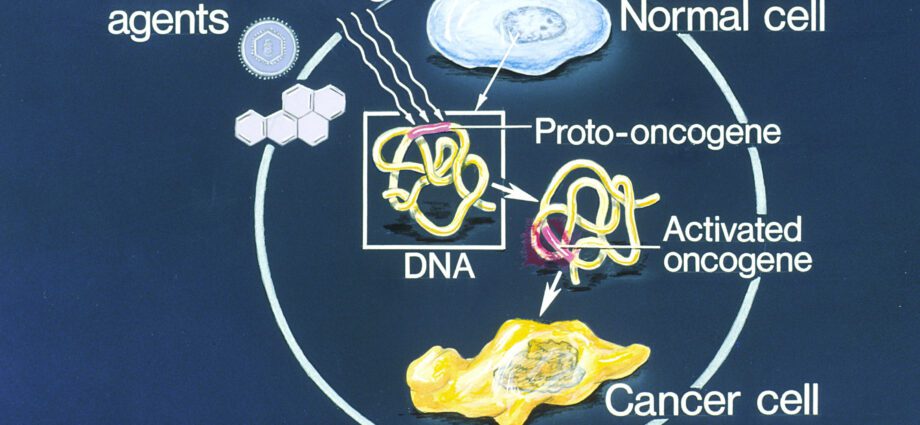
An oncogene is a cellular gene whose expression is likely to promote the development of cancer. What are the different types of oncogenes? By what mechanisms are they activated? Explanations.
What is an oncogene?
An oncogene (from the Greek onkos, tumor and genos, birth) also called proto-oncogene (c-onc) is a gene whose expression is likely to confer a cancerous phenotype on a normal eukaryotic cell. Indeed, oncogenes control the synthesis of proteins that stimulate cell division (called oncoproteins) or inhibit programmed cell death (or apoptosis). Oncogenes are responsible for uncontrolled cell proliferation predisposing to the development of cancer cells.
Oncogenes are divided into 6 classes which correspond respectively to the oncoproteins they encode:
- growth factors. Example: the proto-oncogene encoding proteins of the FGF family (Fibroblast Growth Factor);
- transmembrane growth factor receptors. Example: the proto-oncogene erb B which codes for the EGF (Epidermal Growth Factor) receptor;
- G-proteins or membrane proteins binding GTP. Example: proto-oncogenes of the ras family;
- membrane tyrosine protein kinases;
- membrane protein kinases;
- proteins with nuclear activity.Example: proto-oncogenes erb A, phos, June et c-myc.
What is the role of oncogenes?
Cell renewal is ensured by the cell cycle. The latter is defined by a set of events that generate two daughter cells from a mother cell. We are talking about cell division or “mitosis”.
The cell cycle must be regulated. Indeed, if cell division is not sufficient, the organism does not function optimally; Conversely, if cell division is abundant, cells proliferate uncontrollably, which promotes the appearance of cancer cells.
The regulation of the cell cycle is guaranteed by genes classified into two categories:
- anti-oncogenes which inhibit cell proliferation by slowing the cell cycle;
- proto-oncogenes (c-onc) or oncogenes which promote cell proliferation by activating the cell cycle.
If we compare the cell cycle to a car, the anti-oncogenes would be the brakes and the proto-oncogenes would be the accelerators of the latter.
Anomalies, pathologies linked to oncogenes
The appearance of a tumor may result from a mutation inactivating anti-oncogenes or on the contrary from a mutation activating proto-oncogenes (or oncogenes).
A loss of function of anti-oncogenes prevents them from carrying out their cell proliferation inhibitory activity. The inhibition of anti-oncogenes is the door open to uncontrolled cell division which can lead to the appearance of malignant cells.
However, anti-oncogenes are cellular genes, that is, they are present in duplicate on the pair of chromosomes that carry them in the nucleus of the cell. Thus, when one copy of the anti-oncogene is not functional, the other makes it possible to act as a brake so that the subject is protected against cell proliferation and against the risk of tumors. This is the case, for example, of the BRCA1 gene, the inhibitory mutation of which exposes breast cancer. But if the second copy of this gene is functional, the patient remains protected although he is predisposed due to the defective first copy. As part of such a predisposition, preventive double mastectomy is sometimes considered.
Conversely, the activating mutation affecting proto-oncogenes accentuates their stimulating effect on cell proliferation. This anarchic cell proliferation predisposes to the development of cancers.
Just like anti-oncogenes, pro-oncogenes are cellular genes, present in duplicate on the pair of chromosomes that carry them. However, unlike anti-oncongens, the presence of a single mutated pro-oncogene is sufficient to produce the feared effects (in this case, cell proliferation). The patient carrying this mutation is therefore at risk of cancer.
Mutations in oncogenes can be spontaneous, hereditary or even caused by mutagens (chemicals, UV rays, etc.).
Activation of oncogenes: the mechanisms involved
Several mechanisms are at the origin of activating mutations of oncogenes or pro-oncogenes (c-onc):
- viral integration: insertion of the DNA virus at the level of a regulatory gene. This is for example the case of the human papillomavirus (HPV), which is sexually transmitted;
- point mutation in a sequence of a gene encoding a protein;
- deletion: loss of a larger or smaller fragment of DNA, constituting a cause of genetic mutation;
- structural rearrangement: chromosomal alteration (translocation, inversion) leading to the formation of a hybrid gene encoding a non-functional protein;
- amplification: abnormal multiplication of the number of copies of the gene in the cell. This amplification generally leads to an increase in the level of expression of a gene;
- the deregulation of the expression of an RNA: the genes are disconnected from their normal molecular environment and placed under the inappropriate control of other sequences causing a modification of their expression.
Examples of oncogenes
Genes encoding growth factors or their receptors:
- PDGF: encodes the platelet growth factor associated with glioma (a cancer of the brain);Erb-B: encodes the epidermal growth factor receptor. Associated with glioblastoma (a cancer of the brain) and breast cancer;
- Erb-B2 also called HER-2 or neu: encodes a growth factor receptor. Associated with breast, salivary gland and ovarian cancer;
- RET: encodes a growth factor receptor. Associated with thyroid cancer.
Genes encoding cytoplasmic relays in the stimulation pathways:
- Ki-ras: associated with lung, ovarian, colon and pancreatic cancer;
- N-ras: associated with leukemia.
Genes encoding transcription factors that activate growth-promoting genes:
- C-myc: associated with leukemia and breast, stomach and lung cancer;
- N-myc: associated with neuroblastoma (a cancer of nerve cells) and glioblastoma;
- L-myc: associated with lung cancer.
Genes encoding other molecules:
- Hcl-2: encodes a protein which normally blocks cell suicide. Associated with lymphomas of B lymphocytes;
- Bel-1: also named PRAD1. Encodes Cyclin DXNUMX, a cell cycle clock activator. Associated with breast, head and neck cancer;
- MDM2: encodes an antagonist of the protein produced by the tumor suppressor gene.
- P53: associated with sarcomas (connective tissue cancers) and other cancers.
Focus on ocongene viruses
Oncogenic viruses are viruses that have the ability to make the cell they infect cancerous. 15% of cancers have a viral etiology and these viral cancers are the cause of approximately 1.5 million new cases per year and 900 deaths per year worldwide.
Associated viral cancers are a public health problem:
- the papillomavirus is associated with nearly 90% of cervical cancers;
- 75% of all hepatocarcinomas are linked to hepatitis B and C virus.
There are five categories of oncogenic viruses, whether they are RNA viruses or DNA viruses.
RNA viruses
- Retroviridae (HTVL-1) puts you at risk of T leukemia;
- Flaviviridae (hepatitis C virus) is at risk for hepatocellular carcinoma.
DNA viruses
- Papovaviridae (papillomavirus 16 and 18) exposes to cancer of the cervix;
- Herpesviridae (Esptein Barr virus) exposes to B lymphoma and carcinoma;
- Herpesviridae (human herpesvirus 8) exposes to Kaposi’s disease and lymphomas;
- Hepadnaviridae (hepatitis B virus) is susceptible to hepatocellular carcinoma.