Muscarine (Muscarinum)
This is one of the most poisonous alkaloids, which was discovered by Schmideberg. It was found in the fly agaric Amanita muscaria or Agaricus Muscarius L. From the subfamily of the agaric family Hymenomycetes (Hymenomycetes). Also muscarine has been found in the fungi Boletus luridus and Amanita pantherina and in the fungus Inocybe.
physical properties
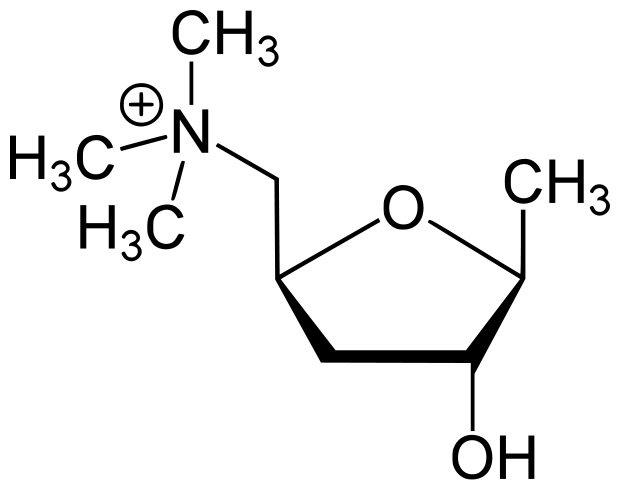
This mushroom-derived alkaloid is called mushroom or natural muscarine, and its empirical formula is C5H15NO8, while no structural formula has been found. Natural muscarine is odorless and tasteless and is a syrupy liquid with a strongly alkaline reaction, which, when dried in the presence of sulfuric acid, gradually turns into a crystalline state. In air, alkaloid crystals spread very quickly, and muscarine reverts to a syrupy liquid. It is highly soluble in alcohol and water, very poorly in chloroform, and completely insoluble in ether. If it is heated above 100 degrees, then it is destroyed, and a not too noticeable smell of tobacco appears. When treated with lead oxide or caustic alkali and heated, it is converted into trimethylamine, and with sulfuric or hydrochloric acid it creates crystalline salts. There is an assumption that the structure of muscarine is similar to the structure of choline (C5H15NO2):
H3C / CH2CH(OH)2
H3C—N
H3C / OH
But the experiments of Schmiedeberg and Harnack show that the artificial alkaloid, obtained synthetically from choline, affects animals differently than the natural one. These experiments showed that artificial and natural muscarines are not identical.
Significance for medicine
Both the natural mushroom alkaloid and the synthetically obtained compound are not currently used for therapeutic purposes, but their medical significance is very high. In former times, attempts were made to treat epilepsy and oncological processes of the glands with muscarine. It was also proposed to be used in eye diseases and for the treatment of ulcers. But all these experiments were stopped due to the exceptional toxicity of the compound.
But muscarine has great toxic, theoretical and pharmacological significance. It belongs to the parasympathicotropic group of poisons, which have a stimulating effect on the peripheral parasympathicotropic nerves, while the alkaloid has a strictly selective effect on the nervous system. This feature makes it of great value as a pharmacological agent that can be used in experiments like electrical stimulation or instead of it.
If in small doses you introduce natural muscarine into the body of an animal, then there is a slowdown in cardiac activity (negative inotropic and chronotropic effects), and in large doses it first causes a slowdown and weakening of systolic contractions. And then in the diastolic phase, a complete cardiac arrest occurs.
Action on the body
Studies by various scientists show that muscarine has a paralyzing effect on the peripheral nervous system of the respiratory tract, causes an increased contraction of the muscles of the stomach and intestines, and the movement of the intestines is visible even through the integuments of the abdominal wall. If muscarine is administered in a large dose, then there are erratic peristaltic movements, which are replaced by antiperistalsis, vomiting and diarrhea begin. A clear sign of muscarine poisoning is the spastic nature of the contractions of the entire stomach or its individual sections, followed by relaxation. According to Schmideberg, muscarine has a very strong effect on the intestines and stomach, not only due to its effect on the endings of the vagus nerves that are located in these organs, but also due to its effect on the ganglion cells of the Auerbach plexus. Also, this alkaloid causes spastic contractions in other smooth muscle organs, for example, in the uterus, spleen and bladder. The contraction occurs as a result of the irritating effect of the substance on the peripheral receptors of the parasympathetic nerves located in these organs, as well as as a result of the influence on the automatic nerve ganglion devices, by analogy with how it happens in the heart. The pupil of the eye under the influence of muscarine is greatly narrowed, a spasm of accommodation develops. These two phenomena are due to the action of the alkaloid on the receptors of the parasympathetic fibers of the oculomotor nerve located in the circular nerves of the iris and in the ciliary muscle.
Schmideberg found that mushroom muscarine does not act on motor nerves, unlike artificial muscarine, which paralyzes motor nerve endings. This was later confirmed by Hans Meyer and Gonda. Thus, curare-like properties are unique to synthetic muscarine derived from choline.
Mushroom muscarine activates the glands of the gastrointestinal tract, stimulates the secretion of bile and pancreatic juice. It also increases salivation, sweating and lacrimation. The secretion of saliva under the action of muscarine is explained by the fact that it irritates the peripheral nerve endings (this was proved by Schmideberg). The secretion of all the other glands is enhanced by the irritating action of muscarine on their scapular nerves. In this case, the target of muscarine action is the peripheral nerve endings.
The direct antagonist of muscarine is atropine, which blocks the effect of muscarine by paralyzing the endings of the parasympathetic nerves. This is manifested in cases where muscarine has an irritating effect on the peripheral receptors of any of the parasympathetic nerves. Therefore, atropine quickly eliminates the diastolic cardiac arrest and slowing of the heart rate provoked by muscarine. Atropine also stops increased peristalsis, antiperistalsis and spasms of the stomach and intestines, accommodation spasm and pupil contraction, bladder contraction, as well as increased secretory function of various glands (sweat, salivary and others). Atropine sulphate exerts its antagonistic effect on muscarine in a rather small amount (0,001-0,1 mg). Muscarine is also known to stop the action of atropine on the frog’s heart, eyes, submandibular gland, and sweat glands. Therefore, there is an opinion that muscarine and atropine are mutual antagonists. But at the same time, a lot of muscarine is required (up to 7 g) in order for the action of atropine to stop. In this regard, it is hardly appropriate to say that muscarine has a specific effect on atropine, and many pharmacologists are of the opinion that the issue of bilateral antagonism of these two compounds has not yet been resolved.
Also, muscarine antagonists include aconitine, hyoscyamine, veratrin, scopolamine, physostigmine, digitalin, delphinium, camphor, helleborine, chloral hydrate, adrenaline. There are interesting facts presented by Tsondek that calcium chloride also has an antagonistic effect on muscarine.
The sensitivity of different animals to muscarine can vary greatly. So the cat dies from the subcutaneous injection of muscarine at a dose of 4 mg after a few hours, and at a dose of 12 mg after 10-15 minutes. Dogs tolerate higher doses of the alkaloid. Humans are very sensitive to this substance. Schmideberg and Koppe conducted experiments on themselves and found that injection of muscarine at a dose of 3 mg already causes poisoning, which is manifested by very strong salivation, rush of blood to the head, dizziness, weakness, redness of the skin, nausea and sharp pains in the abdomen, tachycardia, frustration vision and spasm of accommodation. There is also increased sweating on the face and slightly less on other parts of the body.
Picture of poisoning
In case of mushroom poisoning, the picture may be similar to the description of muscarine poisoning, but usually it still differs due to the fact that fly agaric contains various poisonous atropine-like substances and other compounds that, on the one hand, affect the central nervous system, and on the other hand, stop the action of muscarine . Therefore, poisoning can be characterized by either symptoms from the stomach and intestines (nausea, vomiting, pain, diarrhea) or completely different symptoms, for example, a state of intoxication accompanied by delirium and strong excitement, dizziness, an irresistible desire to destroy everything around, the need to move. Then trembling occurs throughout the body, epileptiform and tetanic convulsions occur, the pupil expands, the rapid pulse becomes much less frequent, breathing is disturbed, becomes irregular, the body temperature drops sharply and a state of collapse develops. In this condition, death occurs in two or three days. In the case of recovery, a person recovers very slowly, a state of hyperleukocytosis is observed in the blood, and the blood itself coagulates very poorly. But to date, there are no reliable and fully confirmed data on blood changes, just as there are no data on pathological changes during poisoning.
First aid
First of all, in case of poisoning with mushrooms, it is necessary to remove the contents from the stomach and intestines. To do this, use emetics, gastric lavage with a probe, and the intestines with an enema. Inside in large doses they drink castor oil. If the symptoms of poisoning characteristic of muscarine predominate, then atropine is injected subcutaneously. If poisoning develops mainly under the influence of atropine-like substances, then atropine cannot be used as an antidote.
Artificial muscarine, which is derived from choline, is the most studied. Very little is known about other artificial muscarines. Anhydromuscarine increases the secretion of sweat and saliva, and has no effect on the eyes and heart. It causes death due to respiratory paralysis. Isomuscarine does not cause cardiac arrest, but slows the heart rate, which can be reversed with atropine. In birds, it leads to a contraction of the pupil, and in mammals it has a curare-like effect on the motor nerves and enhances the secretory function of the glands, does not affect the eyes and intestines, but increases blood pressure. Ptomatomuscarine has a similar effect to cholinemuscarine, which suggests that they have a similar chemical structure. The pharmacological action of uromuscarins has not yet been studied. The same can be said about the pharmacological action of carnomoscarin.









