Contents
Creutzfeldt-Jakob disease
What is it ?
Creutzfeldt-Jakob disease is one of the prion diseases. These are rare diseases characterized by degeneration of the central nervous system and are also called subacute transmissible spongiform encephalopathies (TSE). They are caused by the accumulation in the brain of a normal but poorly conformed protein, the prion protein (1). Unfortunately, Creutzfeldt-Jakob disease is characterized by a rapid and fatal course as well as by the absence of treatment. There are 100 to 150 cases each year in France (2).
Symptoms
The disease often begins with nonspecific disorders such as insomnia or anxiety. Gradually, memory, orientation and language disorders set in. It is then manifested by psychiatric disorders as well as cerebellar ataxia (instability when standing motionless and during walking which is accompanied by a staggering similar to that of drunkenness). There are also typical lesions in the central nervous system (florid plaques, amyloid deposits of PrPres surrounded by vacuoles).
Both sexes are affected, however with a high frequency in young adults.
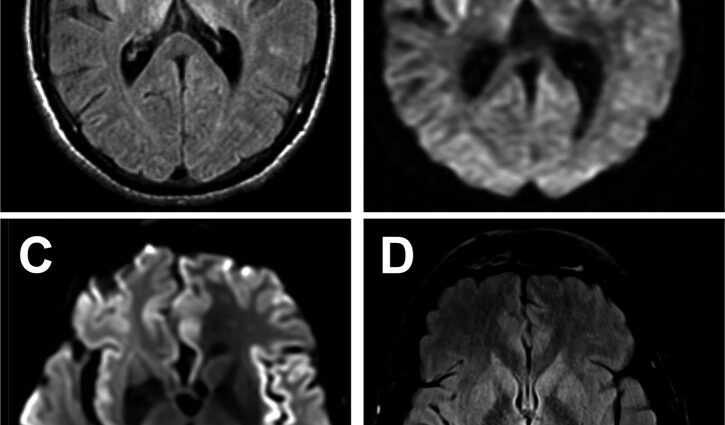
Unfortunately, there is no reliable diagnostic test. An electroencephalogram (EEG) can identify relatively specific disturbances in brain activity. MRI reveals specific abnormalities in certain regions of the brain (basal ganglia, cortex) for which there are few differential diagnoses.
If all of these clinical and paraclinical elements can make it possible to make a diagnosis of Creutzfeldt-Jakob disease, it is only a probable diagnosis: in fact, only the examination of the brain tissue, carried out more often after death allows to confirm the diagnosis.
The origins of the disease
Creutzfeld-Jakob disease is the only human disease that can be of genetic cause (due to a mutation in the gene encoding the prion protein, the E200K mutation being the most common), infectious cause (secondary to contamination) or of sporadic form (of random occurrence, without mutation or exposure to an exogenous prion found).
However, the sporadic form is the most common: it accounts for 85% of all subacute transmissible spongiform encephalopathies (TSEs) diagnosed each year. In this case, the disease usually appears after 60 years and progresses over a period of about 6 months. When the disease is genetic or infectious, the symptoms are earlier and progress more slowly. In infectious forms, the incubation period can be extremely long and exceed 50 years.
Risk factors
The prion protein (PrPc) is a physiological protein that is found in a very conserved manner in many species. In brain neurons, the prion protein can become pathogenic by changing its three-dimensional conformation: it folds up on itself very tightly, which makes it hydrophobic, sparingly soluble and resistant to degradation. It is then called the “scrapie” prion protein (PrPsc). PrPsc aggregate with each other and form deposits that multiply inside and outside brain cells, disrupting their function and survival mechanisms.
In this abnormal form, the prion protein is also capable of transmitting its conformational anomaly: on contact with a PrPsc, a normal prion protein in turn adopts an abnormal conformation. This is the domino effect.
The risk of transmission between individuals
Interindividual transmission of prion diseases is possible with tissue transplantation or following the administration of growth hormones. The most risky tissues come from the central nervous system and the eye. To a lesser extent, cerebrospinal fluid, blood and certain organs (kidneys, lungs, etc.) can also transmit the abnormal prion.
The risk of food
The transmission of a prion from cattle to humans via the consumption of contaminated food was suspected in 1996, during the dramatic “mad cow” crisis. For several years now, an epidemic of bovine spongiform encephalopathy (BSE) has hit herds in the United Kingdom3. The spread of this prion disease, which affected tens of thousands of animals each year, was undoubtedly due to the use of animal meal, produced from carcasses and insufficiently decontaminated. Its origin, however, remains debated.
Prevention and treatment
Today, there is no specific treatment for prion diseases. The only drugs that can be prescribed are those that can relieve or limit the various symptoms of the disease. Medical, social and psychological support is offered to patients and their families by the National CJD Support Unit. The search for drugs aimed at preventing the conversion of PrPc, promoting the elimination of abnormal forms of the protein and limiting its spread is hopeful. An interesting lead targets PDK1, one of the cellular mediators involved during infection. Its inhibition would make it possible both to inhibit the conversion phenomenon by promoting the cleavage of PrPc, and to attenuate the consequences of its replication on the survival of neurons.