Contents
Vaxigrip is a flu vaccine that can be vaccinated in both adults and children from six months of age. The purpose of the vaccine is to protect the person vaccinated against the flu. After it is administered, the human natural immune system begins to produce protection against the flu, i.e. antibodies. Importantly, the Vaxigrip Tetra vaccine complies with the recommendations of the World Health Organization, i.e. WHO, and the recommendations of the European Union.
Vaxigrip – composition
Vaxigrip is a suspension for injection designed to prevent flu disease. After injection of the vaccine, the patient develops an immune response that triggers the development of antibodies against the influenza virus antigens in the vaccine.
The vaccine contains inactivated influenza viruses belonging to 4 strains, which were propagated in chicken embryos. Vaxigrip is a guarantee of building immunity against these 4 strains of influenza virus, therefore it is referred to as 4-valent. By vaccinating Vaxigrip Tetra, we gain immunity to 2 A and 2 B subtypes of the influenza virus.
Vaxigrip Tetra as a 4-valent vaccine is much more effective because it is never certain which type of virus we will be exposed to in the next disease season. In addition, as the influenza virus is highly variable, it is recommended that Vaxigrip Tetra is vaccinated annually. In addition, to increase the effectiveness of Vaxigrip each year, a new flu vaccine is being developed that contains different strains of the flu virus as determined by specialists for that year. Each new version of the vaccine complies with the recommendations of the WHO and the European Union for a given season.
Vaxigrip – indications
Vaxigrip is recommended to be used in the case of:
- active immunization against influenza children after 6 months of age, as well as adults, including pregnant women;
- passive flu immunization infants from birth to 6 months of age, if a pregnant woman is vaccinated.
Check it out: How to cure the flu?
Vaxigrip – contraindications
Keep in mind that even when there are indications for Vaxigrip, it may not always be used. There are situations that may be a complete contraindication to the use of Vaxigrip. The main contraindication to the administration of the Vaxigrip vaccine is hypersensitivity, i.e. allergy to any of the ingredients of the preparation. Caution should also be exercised in the case of the ingredients of Vaxigrip, which are present in the preparation in trace amounts and are a residue of the production of the vaccine. These are i.a. ovalbumin, neomycin, egg white, formaldehyde and octoxynol-9.
A periodic contraindication to vaccination with Vaxigrip is an acute illness or a disease with moderate or high fever. Vaccination should then be postponed until symptoms have resolved.
In addition, it is very important not to administer the vaccine intravascularly.
Vaxigrip – precautions
It should be remembered that some diseases or other circumstances related to our health may constitute a contraindication to administering Vaxigrip or changing the dose of the preparation. In certain situations, it may be necessary to perform specific check-ups from time to time.
Vaxigrip may only be administered by qualified personnel in clinics and hospitals. It is very important that the preparation is administered subcutaneously or intramuscularly, not intravascularly. It is also imperative that the patient be provided prompt medical attention in the event of a severe anaphylactic reaction following an injection of Vaxigrip. Although severe anaphylactic reactions (shock) are rare, they can be directly life-threatening. If this occurs, immediate treatment is required.
In addition, particular care should be taken when administering the vaccine to people who have:
- thrombocytopenia;
- blood coagulation disorders.
In this group of people, bleeding may occur after intramuscular injection of Vaxigrip.
Sensitive people after the injection of Vaxigrip, and even before the injection is given, the so-called psychogenic reaction as a result of a needle stick injury. You may then faint. People who suspect that such a reaction may occur should inform the medical staff at the medical facility so that they can prevent possible injury from fainting.
Before deciding to vaccinate against flu, remember that the vaccine will only protect your body against the flu strains that were used to make it. In addition, as with any vaccine, Vaxigrip may not be effective in protecting everyone who is vaccinated against getting the flu. This is especially true for people with acquired or congenital immunodeficiency, where the immune response may not be sufficient.
It is also important to know that not all infants up to the age of 6 months, whose mothers received Vaxigrip during pregnancy, will acquire passive immunity against the flu and will be protected against the virus.
We should also remember that vaccination may affect the results of serological tests using the ELISA method, so you should inform your doctor about the vaccination before performing serological tests.
In addition, Vaxigrip may contain traces of chicken protein, ovalbumin, formaldehyde, octoxynol-9 and neomycin. Vaxigrip has no influence on the ability to drive and use machines.
Check it out: Cold or Flu?
Vaxigrip – dosage
Vaxigrip is a suspension suitable for injection in a pre-filled syringe. It is a preparation intended only for intramuscular or subcutaneous injections. Remember to consult your doctor about any doubts related to the method of administration of the preparation. Vaccination with Vaxigrip is recommended each year.
Dosage Vaxigrip:
- adults and children over 6 months of age – one dose of 0,5 ml is administered once a year;
- unvaccinated children up to 9 years of age should take another dose 4 weeks after the first injection within 4 weeks.
There are no data on the safety and efficacy of the preparation in children under 6 months of age.
For passive immunization, a pregnant woman should be given 1 dose of 0,5 ml of Vaxigrip. Then, the vaccine administered in this way may protect the child from catching the flu until the age of 6 months. However, it should be remembered that not all babies will be protected in this way.
Vaxigrip – administration
Vaxigrip is administered intramuscularly or subcutaneously. The preparation should not be administered intravascularly.
It is best to administer the preparation intramuscularly to:
- the anterolateral part of the thigh or the deltoid muscle, if the muscle mass is sufficient – this one method of administration of Vaxigrip it is preferred in both infants after 6 months of age and adults up to 35 years of age;
- the deltoid muscle in children over 36 months of age and in adults.
Check it out: Should my child get the flu vaccine?
Vaxigrip and pregnancy and breastfeeding
Pregnant and breastfeeding women should not take any medications without consulting a doctor. In the case of pregnant and breastfeeding women, it is very important that they consult a doctor before taking any preparation to explain any possible side effects and the benefits of its administration. Therefore, in any case, pregnant and breastfeeding women should inform the prescribing physician about this fact.
Pregnant women should consult their doctor before vaccination. At the same time, it is assumed that pregnancy at any stage is not a contraindication to receiving an inactivated influenza vaccine. Likewise, Vaxigrip can be taken by breastfeeding women.
Vaxigrip – interactions with other drugs
Before vaccinating, inform your doctor about all medications you have recently taken, including those obtained without a prescription. It’s also worth knowing that there are no data available on the safety of using Vaxigrip with other vaccines. It has been accepted that Vaxigrip can be used concurrently with other vaccines. However, if we decide on such a solution, the vaccine should not be administered twice to the same place and, of course, it is necessary to change the syringes each time.
It should also be remembered that people undergoing immunosuppressive treatment may have a lower immune system response and influenza vaccination itself may affect the results of serological tests using the ELISA method.
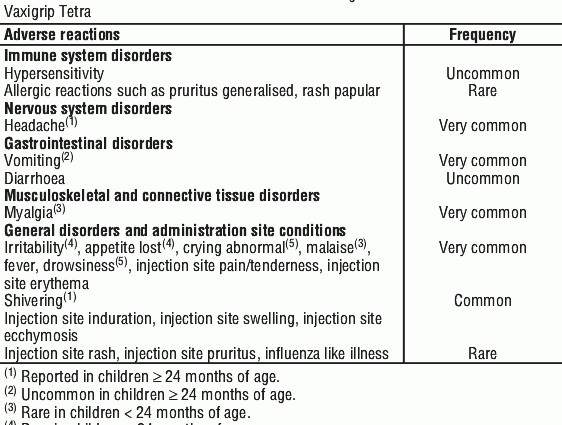
Vaxigrip – side effects
Vaxigrip, like any other drug, may cause side effects. However, it should be remembered that they do not occur in everyone, and most often the expected benefits of taking the drug are higher than the possible harm as a result of side effects.
After vaccination, side effects can be observed after approx. 3 days, and, importantly, they disappear on their own after approx. 1-3 days after the onset. Side effects with Vaxigrip are often mild. The most common are:
- edema;
- erythema;
- pain and thickening at the injection site;
- muscle aches;
- chills and fever;
- headaches;
- declines in well-being.
You can also expect:
- adults and the elderly – uncommon: swollen lymph nodes, hot flushes, dizziness, nausea and diarrhea, bruising, weakness, itching at the injection site and a feeling of warmth; less frequently: paresthesia, somnolence, hyperhidrosis, dyspnoea, weakness, joint pain, hypersensitivity reactions, flu-like symptoms;
- children over 3 years of age and adolescents – uncommon: whining, anxiety, thrombocytopenia, dizziness, vomiting, diarrhea, epigastric and joint pain, warmth and itching at the injection site and fatigue;
- children between 6 and 36 months of age – very often irritability, vomiting, lack of appetite, unusual crying, often chills, great drowsiness; hypersensitivity and diarrhea are uncommon; allergic reactions and flu-like symptoms are rare.
In addition, post-injection patients reported transient thrombocytopenia, enlarged lymph nodes, severe allergic and anaphylactic reactions, rashes, generalized erythema, neuritis, convulsions, encephalomyelitis, Guillain-Barré syndrome, paraesthesia, vasculitis, occasionally with intermittent disorders. kidney function.