Contents
The purpose of the article is to analyze the theoretical and some practical aspects of the work of a home distillation column aimed at obtaining ethyl alcohol, as well as dispel the most common myths on the Internet and clarify the points that equipment sellers are “silent about”.
Rectification of alcohol – separation of a multicomponent alcohol-containing mixture into pure fractions (ethyl and methyl alcohols, water, fusel oils, aldehydes, and others) having different boiling points by repeated evaporation of the liquid and condensation of steam on contact devices (plates or nozzles) in special counterflow tower apparatuses.
From a physical point of view, rectification is possible, since initially the concentration of individual components of the mixture in the vapor and liquid phases is different, but the system tends to equilibrium – the same pressure, temperature and concentration of all substances in each phase. Upon contact with a liquid, the vapor is enriched with volatile (low-boiling) components, while the liquid, in turn, is enriched with low-volatile (high-boiling) components. Simultaneously with enrichment, heat exchange takes place.
The moment of contact (interaction of flows) between vapor and liquid is called the process of heat and mass transfer.
Due to the different directions of movements (steam rises, and the liquid flows down), after the system reaches equilibrium in the upper part of the distillation column, it is possible to separately select practically pure components that were part of the mixture. First, substances with a lower boiling point (aldehydes, esters and alcohols) come out, then with a high one (fusel oils).
A state of balance. Appears at the very boundary of the phase separation. This can only be achieved if two conditions are met simultaneously:
- Equal pressure of each individual component of the mixture.
- The temperature and concentration of substances in both phases (vapor and liquid) is the same.
The more often the system comes into equilibrium, the more efficient the heat and mass transfer and the separation of the mixture into individual components.
Difference between distillation and rectification
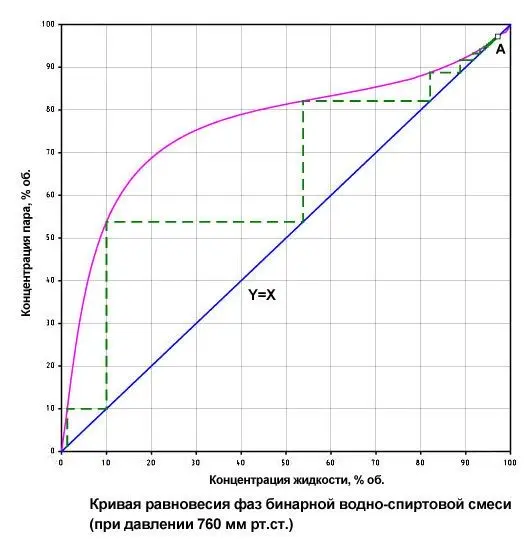
As you can see in the graph, from a 10% alcohol solution (mash) you can get 40% moonshine, and during the second distillation of this mixture, a 60-degree distillate will come out, and during the third – 70%. The following intervals are possible: 10-40; 40-60; 60-70; 70-75 and so on up to a maximum of 96%.
Theoretically, to get pure alcohol, 9-10 successive distillations are required on a moonshine still. In practice, it is explosive to distill alcohol-containing liquids with a concentration above 20-30%, and besides, due to the high energy and time costs, it is economically unprofitable.
From this point of view, the rectification of alcohol is a minimum of 9-10 simultaneous, stepwise distillations that occur on different contact elements of the column (packings or plates) along the entire height.
| Distinction | Distillation | Rectification |
| Organoleptics of the drink | Keeps aroma and taste of initial raw materials. | It turns out pure alcohol without smell and taste (the problem has a solution). |
| Fortress at the exit | Depends on the number of distillations and the design of the apparatus (usually 40-65%). | Up to 96%. |
| The degree of separation into fractions | Low, substances even with different boiling points are mixed, it is impossible to fix this. | High, pure substances can be isolated (only with different boiling points). |
| Ability to remove harmful substances | Low or medium. To improve the quality, a minimum of two distillations with separation into fractions in at least one of them is required. | High, with the right approach, all harmful substances are cut off. |
| Alcohol loss | High. Even with the right approach, you can extract up to 80% of the total amount, while maintaining an acceptable quality. | Low. Theoretically, it is possible to extract all the ethyl alcohol without loss of quality. In practice, at least 1-3% loss. |
| The complexity of the technology for implementation at home | Low and medium. Even the most primitive apparatus with a coil is suitable. Equipment improvements are possible. The technology of distillation is simple and clear. A moonshine still does not usually take up much space in working order. | High. Special equipment is required, which is impossible to manufacture without knowledge and experience. The process is more difficult to understand, preliminary at least theoretical preparation is needed. The column takes up more space (especially in height). |
| Danger (compared to each other), both processes are flammable and explosive. | Due to the simplicity of the moonshine still, distillation is somewhat safer (subjective opinion of the author of the article). | Due to the complex equipment, when working with which there is a risk of making more mistakes, rectification is more dangerous. |
Operation of the distillation column
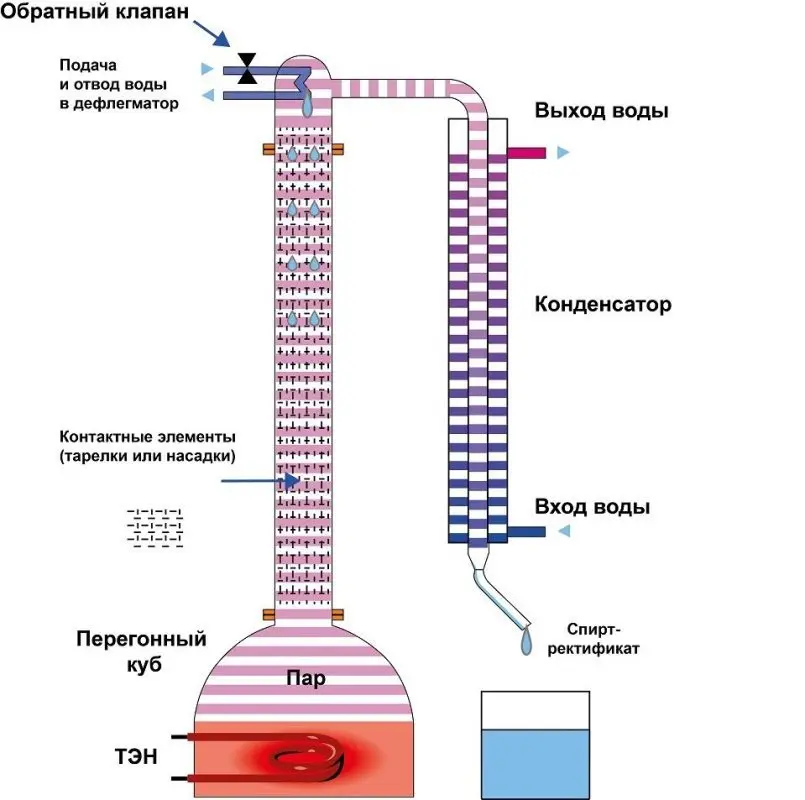
Distillation column – a device designed to separate a multicomponent liquid mixture into separate fractions according to the boiling point. It is a cylinder of constant or variable section, inside which there are contact elements – plates or nozzles.
Also, almost every column has auxiliary units for supplying the initial mixture (raw alcohol), controlling the rectification process (thermometers, automation) and distillate extraction – a module in which the vapor of a certain substance extracted from the system is condensed and then taken out.
Raw alcohol – a product of the distillation of mash by the classical distillation method, which can be “filled” into a distillation column. In fact, this is moonshine with a strength of 35-45 degrees.
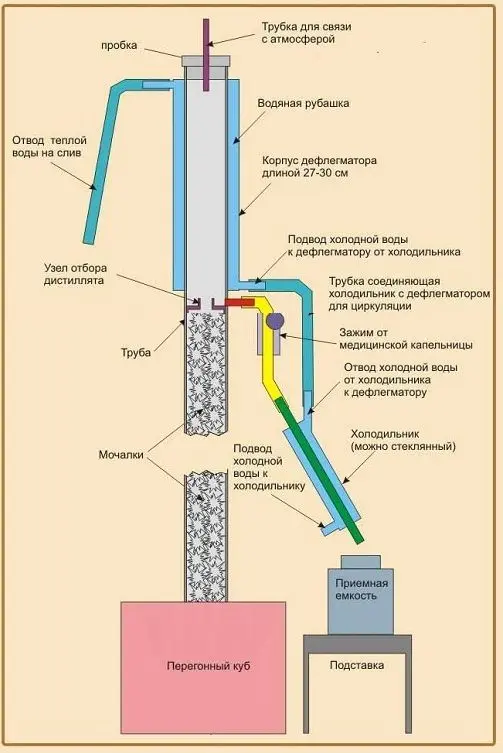
Phlegm – steam condensed in the dephlegmator, flowing down the walls of the column.
Phlegm number – the ratio of the amount of reflux to the mass of the sampled distillate. There are three streams in the alcohol distillation column: steam, phlegm and distillate (end goal). At the beginning of the process, the distillate is not withdrawn so that there is enough reflux in the column for heat and mass transfer. Then part of the alcohol vapor is condensed and taken from the column, and the remaining alcohol vapor continues to create a reflux flow, ensuring normal operation.
For the operation of most installations, the reflux ratio must be at least 3, that is, 25% of the distillate is taken, the rest is needed in the column for irrigating the contact elements. As a general rule, the slower the alcohol is withdrawn, the higher the quality.
Distillation column contact devices (trays and packings)
Responsible for the multiple and simultaneous separation of the mixture into liquid and vapor, followed by condensation of the vapor into a liquid – the achievement of an equilibrium state in the column. Ceteris paribus, the more contact devices in the design, the more effective distillation in terms of alcohol purification, since the surface of interaction of phases increases, which intensifies the entire heat and mass transfer.
theoretical plate – one cycle of exit from the equilibrium state with its repeated achievement. To obtain high-quality alcohol, a minimum of 25-30 theoretical plates is required.
physical plate – a real working device. The vapor passes through the liquid layer in the plate in the form of many bubbles, creating an extensive contact surface. In the classical design, the physical plate provides about half of the conditions for reaching one equilibrium state. Therefore, for the normal operation of the distillation column, two times more physical plates are required than the theoretical (calculated) minimum – 50-60 pieces.
Nozzles Often, plates are placed only on industrial installations. In laboratory and home distillation columns, nozzles are used as contact elements – specially twisted copper (or steel) wire or dishwashing nets. In this case, the phlegm flows down in a thin stream over the entire surface of the nozzle, providing the maximum contact area with steam.

There are a lot of structures. The disadvantage of home-made wire nozzles is the possible damage to the material (blackening, rust), factory counterparts are devoid of such problems.
Properties of distillation column
Material and dimensions. The column cylinder, nozzles, cube and distillers must be made of a food-grade, stainless, heat-safe (expands evenly) alloy. In home-made designs, cans and pressure cookers are most often used as a cube.
The minimum length of the pipe of a home distillation column is 120-150 cm, diameter is 30-40 mm.
heating system. In the process of rectification, it is very important to control and quickly adjust the heating power. Therefore, the most successful solution is heating with the help of heating elements built into the bottom of the cube. The supply of heat through a gas stove is not recommended, since it does not allow you to quickly change the temperature range (high inertia of the system).
Process control. During rectification, it is important to follow the instructions of the column manufacturer, which must indicate the features of operation, heating power, reflux ratio and model performance.

It is very difficult to control the rectification process without two simple devices – a thermometer (helps determine the correct degree of heating) and an alcohol meter (measures the strength of the resulting alcohol).
Performance. It does not depend on the size of the column, since the higher the side (pipe), the more physical plates are inside, therefore, the cleaning is better. The performance is affected by the heating power, which determines the speed of steam and reflux flows. But with an excess of supplied power, the column chokes (stops working).
The average productivity of home distillation columns is 1 liter per hour with a heating power of 1 kW.
Influence of pressure. The boiling point of liquids depends on pressure. For successful distillation of alcohol, the pressure at the top of the column should be close to atmospheric – 720-780 mm Hg. Otherwise, when the pressure decreases, the vapor density will decrease and the evaporation rate will increase, which may cause flooding of the column. If the pressure is too high, the evaporation rate drops, making the operation of the device inefficient (no separation of the mixture into fractions). To maintain the correct pressure, each distillation column is equipped with an atmospheric connection tube.
About the possibility of self-made assembly. Theoretically, a distillation column is not a very complex device. Designs are successfully implemented by craftsmen at home.
But in practice, without understanding the physical foundations of the rectification process, correct calculations of equipment parameters, selection of materials and high-quality assembly of units, the use of a home-made distillation column turns into a dangerous occupation. Even one mistake can lead to fire, explosion or burns.
In terms of safety, factory columns that have been tested (have supporting documentation) are more reliable, and they are also supplied with instructions (must be detailed). The risk of a critical situation comes down to only two factors – proper assembly and operation according to the instructions, but this is a problem for almost all household appliances, and not just columns or moonshine stills.
The principle of operation of the distillation column
The cube is filled with a maximum of 2/3 of the volume. Before turning on the installation, it is imperative to check the tightness of the connections and assemblies, shut off the distillate extraction unit and supply cooling water. Only after that you can start heating the cube.
The optimal strength of the alcohol-containing mixture fed into the column is 35-45%. That is, in any case, distillation of the mash is required before rectification. The resulting product (raw alcohol) is then processed on a column, obtaining almost pure alcohol.
This means that a home distillation column is not a complete replacement for the classic moonshine still (distiller) and can only be considered as an additional purification step that replaces re-distillation (second distillation) in a better quality, but leveling the organoleptic properties of the drink.
In fairness, I note that most modern models of distillation columns involve working in the moonshine still mode. To proceed to distillation, it is only necessary to close the connection to the atmosphere and open the distillate selection unit.
If both nozzles are closed at the same time, then the heated column may explode due to excess pressure! Do not make such mistakes!
In continuous industrial plants, mash is often distilled immediately, but this is possible due to its gigantic size and design features. For example, a pipe 80 meters high and 6 meters in diameter is considered a standard, in which many more contact elements are installed than on distillation columns for a house.

After switching on, the liquid in the cube is brought to a boil by the heater. The resulting steam rises up the column, then enters the reflux condenser, where it condenses (phlegm appears) and returns in liquid form to the lower part of the column along the pipe walls, on the way back contacting the rising steam on plates or nozzles. Under the action of the heater, the phlegm again becomes steam, and the steam at the top is again condensed by a dephlegmator. The process becomes cyclic, both streams are in continuous contact with each other.
After stabilization (steam and phlegm are sufficient for an equilibrium state), pure (separated) fractions with the lowest boiling point (methyl alcohol, acetaldehyde, ethers, ethyl alcohol) accumulate in the upper part of the column, with the highest (fusel oils) at the bottom. As the selection of the lower fractions gradually rise up the column.
In most cases, a column in which the temperature does not change for 10 minutes (total warm-up time is 20-60 minutes) is considered stable (it is possible to start sampling). Up to this point, the device works “on its own”, creating flows of steam and phlegm that tend to balance. After stabilization, the selection of the head fraction containing harmful substances begins: esters, aldehydes and methyl alcohol.
The distillation column does not eliminate the need to separate the output into fractions. As in the case of a conventional moonshine still, you have to assemble the “head”, “body” and “tail”. The difference is only in the purity of the output. During rectification, the fractions are not “lubricated” – substances with a close, but at least a tenth of a degree, different boiling point do not intersect, therefore, when the “body” is selected, almost pure alcohol is obtained. During conventional distillation, it is physically impossible to separate the yield into fractions consisting of only one substance, no matter what design is used.
If the column is brought to the optimal mode of operation, then there are no difficulties during the selection of the “body”, since the temperature is stable all the time.
The lower fractions (“tails”) are selected during rectification, guided by temperature or smell, but unlike distillation, these substances do not contain alcohol.
Return of organoleptic properties to alcohol. Often, “tails” are required to return the “soul” to rectified alcohol – the aroma and taste of the raw material, for example, apples or grapes. After the process is completed, a certain amount of collected tail fractions is added to pure alcohol. The concentration is calculated empirically by experimenting on a small amount of product.
The advantage of rectification is the ability to extract almost all the alcohol contained in the liquid without losing its quality. This means that the “heads” and “tails” obtained on a moonshine still can be processed on a distillation column and ethyl alcohol safe for health can be obtained.
Flooding of distillation column
Each design has a maximum speed of steam movement, after which the flow of phlegm in the cube first slows down, and then stops altogether. The liquid accumulates in the distillation part of the column and “flooding” occurs – the termination of the heat and mass transfer process. Inside there is a sharp pressure drop, extraneous noise or gurgling appears.
Causes of flooding of the distillation column:
- exceeding the permissible heating power (most common);
- clogging the bottom of the device and overflowing the cube;
- very low atmospheric pressure (typical for high mountains);
- the voltage in the network is higher than 220V – as a result, the power of the heating elements increases;
- design errors and failures.









