Contents
Maintaining the optimum distillation temperature results in crystal clear moonshine without odor and harmful impurities. This is one of the most important stages of home brewing, without knowing the basics of which you cannot count on a good result. Without observing the distillation technology, even the best home brew will turn out to be bad moonshine.
Theoretical aspects
Boiling point and volatility of impurities
The most common misconception among beginner moonshiners is that impurities evaporate in proportion to their boiling point. In fact, this is fundamentally not the case: the volatility of impurities, that is, their ability to leave a boiling liquid, is in no way related to the boiling points of these impurities.
Consider the classic example of methanol and isoamylol. Let the raw material of the following composition be poured into the cube (see table).
| The composition of the distilled liquid | The boiling point of substances (°C) | Ratio in solution (%) |
| water | 100 | 86 |
| ethanol | 78 | 12 |
| Methanol | 65 | 1 |
| isoamylol | 132 | 1 |
Bring the mixture to a boil (temperature in the cube is about 92 ° C) and select a small amount of distillate so that the composition of the boiling raw material remains practically unchanged. What will be the composition of the selected distillate? For water and ethyl alcohol, the change in concentrations can be easily found from the equilibrium curve or tables: the alcohol concentration will increase from 12 to 59%.
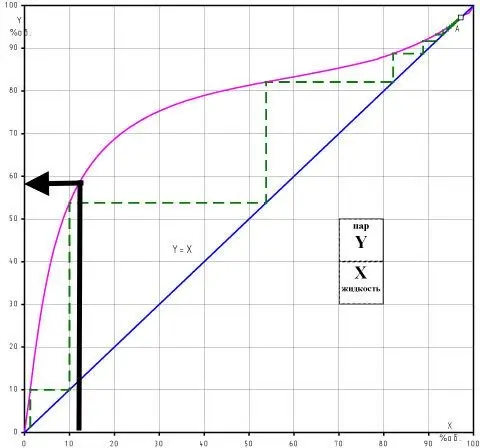
To determine the change in the concentration of impurities, we use the graph of the rectification coefficients (strength as a percentage of the volume is on the upper horizontal axis).
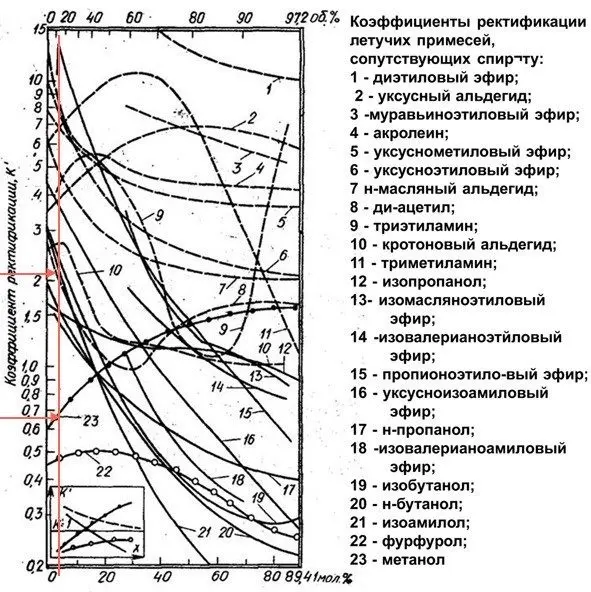
With a raw material strength of 12%, the rectification coefficient (Kp) of methyl alcohol is 0,67, and Kp of isoamylol is 2,1. This means that the content of methanol in the selection will decrease, and isoamylol will double. The result is.
| The composition of the distilled liquid | The boiling point of substances (°C) | Ratio in solution (%) | Quantity change | |
| in raw materials | in distillate | |||
| water | 100 | 86 | 38.2 | less than 2,2 times |
| ethanol | 78 | 12 | 59 | 4,9 times more |
| Methanol | 65 | 1 | 0.67 | less than 1,5 times |
| isoamylol | 132 | 1 | 2.1 | 2,1 times more |
The second table proves the independence of the evaporation rate of impurities from their boiling point. Methanol with a boiling point of 65 ° C leaves the cube more slowly than isoamylol with a boiling point of 132 degrees.
This is because the concentration of these impurities is low. If the amount of methanol and isoamylol were comparable to alcohol and water, these substances would declare their right to evaporate in an amount corresponding to the difference in their boiling points, and would become full-fledged components of the solution.
The volatility of impurities at a concentration of less than 2% depends entirely on the strength with which their single molecules are retained by a water-alcohol solution (substances predominant in the composition). This can be compared to how dad and mom do not ask the child at what speed to run to the bus – they took hands and galloped.
Same with impurities. When in solution one small molecule of methanol is surrounded by a crowd of water molecules, they easily keep it next to them. Since the methanol molecule is smaller than ethanol, it is much easier for water to hold it. But isoamylol, on the contrary, is poorly soluble in water, having very weak bonds with it. When boiling, isoamylol flies out of water faster than methanol, although its boiling point is 2 times higher.
Sorel devoted many of his works to the study of the coefficients of evaporation or volatility of various substances and their solutions. He compiled tables and graphs by which one can find out how much the content of substances in vapors changes in relation to the initial solution. However, for the purposes of distillation, graphs and tables are inconvenient to use, so Barbe proposed a new calculation coefficient, called the rectification coefficient (Kp), to obtain which, for a given strength of the solution, it is necessary to divide the evaporation coefficient of the impurity by the evaporation coefficient of ethyl alcohol.
The rectification coefficient is also the purification coefficient, as it shows the actual change in the content of impurities in relation to ethyl alcohol:
- Kp = 1 – impurities cannot be eliminated, they will be present in the distillate in the same amount;
- Kr>1 – there will be more impurities in the selection than in the feedstock, these are head fractions;
- Кр
If impurities at high alcohol concentrations have Kp1, these are intermediate impurities. These are the vast majority. There are also terminal impurities, which, on the contrary, have Kp>1 at a high alcohol concentration, and at a low one – Kp
In fact, there are not so many absolutely head or tail impurities, more often distillers deal with intermediate ones. However, if we talk about the distillation of mash, then its fortress changes during the process from 12% and below. At such concentrations of alcohol, almost all impurities are head impurities, regardless of their boiling point: isoamylol – 132 °C, acetaldehyde – 20 °C, etc.
There are very few impurities that exhibit tailing properties during the distillation of mash: methanol with a boiling point of 65 degrees and furfural – 162 ° C. As you can see, here the boiling point does not affect anything.
Main theoretical conclusion. Impurities do not line up to leave the cube in accordance with their boiling points, but evaporate as part of the alcohol vapor in quantities that depend only on their initial concentration and rectification coefficient.
Heating power and solution boiling point
The heating power only affects the amount of steam generated and does not change the boiling point of the contents of the cube. In turn, the boiling point of the solution depends on the concentration of alcohol in the bottom bulk and atmospheric pressure (see table).
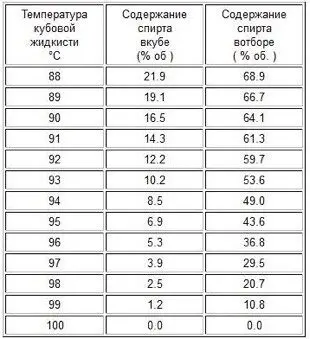
The lower the fortress, the higher the boiling point of the cubic bulk. The more power supplied, the more steam is generated.
Fractional distillation
If, when the mixture is boiled on the way to the refrigerator, its vapors do not condense on the lid and walls of the cube, or this value is negligible, then by selecting the shoulder strap sequentially in different cans, we get different strength and composition of the distillate in them.
This is a simple fractional distillation, which can only be controlled conditionally by changing the proportions of the selected fractions. The method does not provide for any cleaning or strengthening.
If the apparatus is ideally insulated, then regardless of the extraction rate and heating power, the output will be a distillate of the same composition and strength.
Partial condensation
If a noticeable part of the steam condenses on the way from the cube to the refrigerator, this is partial condensation.
The walls of the cube, the lid and the steam pipe continuously lose heat. These heat losses do not depend on the amount of heating or extraction, but only on the temperature difference between the bottoms (liquid and steam) and the surrounding air.
The consequence of this process, useful in distillation, is the partial condensation of steam, when its least volatile components enter the phlegm, which then flow back into the cube.
The same part of the steam that reaches the refrigerator contains more volatile components than it was in the original pairs. This allows you to create conditions for a more concentrated selection of “heads” and strengthen the selection.
The ratio of the weight of reflux to the weight of the selected alcohol is called the phlegm number. The higher the reflux number, the greater the strengthening and enrichment with volatile selection components.
It is also important to note that the phlegm flowing into the cube warms up, causing additional condensation of steam, but does not have time to boil.
Heat transfer
If the phlegm flows into the cube for so long that the steam has time to warm it to the boiling point, another process occurs – heat and mass transfer, in which molecules of non-volatile substances condense from the steam, and volatile substances evaporate from the phlegm. Evaporate and condense always an equal number of molecules. This process underlies the rectification technology.
How to drive moonshine on a conventional machine
Having become acquainted with some questions of theory, we can proceed to the question of controlling the distillation process.
Apparatus for classical distillation are built according to the cube-refrigerator scheme. Adding a dryer makes it easier to take off the “body” at high speeds, as it prevents splashing. The cube and steam pipes are not insulated, and as we will find out later, it is no coincidence. Distillers may be different (see photo).

Fundamentally, these devices differ only in the degree of partial condensation. With its small proportion, the apparatus is suitable only for the distillation of mash, with a large partial condensation it is suitable for the production of noble distillates.
Distillation of brew
Braga needs to be driven quickly. The main task is to separate all evaporable components from non-evaporable ones. Power reduction at the beginning or at the end of heating is not required. At the first distillation of mash on alambika, it is advisable to cover its dome with a rag.
Ordinary sugar mash can be selected “dry” (minimum strength in the stream). In the case of fruit brews that are planned to be aged in barrels, it is advisable to drive up to an average strength of 25%. If you finish the process earlier, acids and heavy alcohols will be lost, which form new esters in the cask.
Second distillation
Bulk fortress. The optimal strength of the bottom liquid for the second stage is 25-30%. With such a concentration of alcohol, the fuselage is sufficiently well strengthened and excreted as part of the head fraction. An acceptably small proportion of alcohol will get into the “tails”, but when selecting the “body”, it will not be possible to keep the fusel in the cube or a reflux ratio of more than 3 will be required, which will seriously delay the distillation process, and not every apparatus can operate in this mode.
The lower initial strength of the bulk will allow the sivukha during the selection of “heads” to come out with a concentration higher than the vat more than twice, but the selection of the “body” will begin at a too low strength of the bulk, as a result, almost half of the alcohol will fall into the “tails”, which need to be started select at the strength of the liquid in the cube 5-10%.
If you increase the strength of the cubic bulk to 35-40% or more, then the strengthening of the fusel oil at low reflux numbers will not occur. In the “heads” there will be as much fusel as in the still, and with drip selection (increase in reflux numbers), the fusel will generally remain in the cube.
The selection of the “body” will take place with less loss of alcohol in the “tails”, but all the remaining fusel in the cube will fall into the “body”. Due to the fact that the volume of alcohol in the selection will decrease, the concentration of fusel oil will be even greater than in the bulk.
Head selection. Consider what happens when selecting “heads” on a classic moonshine still. For example, a vat bulk with a strength of 25-30% boiled, and the distiller reduced the heating power to 600 watts. In this case, the heat loss of the steam zone is 300 W (we will neglect the heat loss in the liquid zone for simplicity of calculations). As a result, exactly half of the steam formed in the cube will condense. The amount of selection will be equal to the amount of phlegm, which means that the phlegm number is equal to one. Increasing the heating power will lead to a decrease in the reflux ratio and, conversely, a further decrease in power will increase it.
When organizing drop-by-drop selection of “heads”, the system reaches the maximum phlegm number, which strengthens and enriches the selection with volatile impurities.
During distillation, the bulk has a low strength, and almost all impurities are head. Therefore, the selection of “heads” is extremely important, it is necessary to create conditions for its successful implementation:
- always leave a sufficiently large steam zone in the cube, and not chase the bulk volume;
- do not insulate the cube with a lid and the steam pipe of the distiller.
Getting a “body” The rate of removal of the “body” in the second fractional distillation should be moderate so as not to minimize the reflux ratio.
Most household classic devices do not have sufficient capabilities for partial condensation, so there are only two ways to get an acceptable cleaning of the “body” on them: remove impurities with “heads” or cut them off with “tails”.
When to collect tails. It is widely believed that the moment to switch to the selection of “tails” comes when the fortress in the jet is 40%, has solid ground under it.
Intermediate impurities increase their rectification coefficient to values exceeding one, and become an easily volatile component of the vapor, which means that they no longer go into phlegm, but continue on their way to selection. Condensation is mainly water and typically tail impurities. Partial condensation ceases to purify alcohol vapor from the fuselage, but, on the contrary, enriches it.
At the time of tailings sampling, the bottom temperature is about 96 °C, which corresponds to a bottom strength of about 5%. “Tails” can be taken up to 98-99 degrees in a cube, it is not necessary to dry completely, too many impurities and water will appear.
As an alternative, I recommend that you familiarize yourself with other methods for separating distillate into fractions, which are suitable even for devices without a thermometer.
Distillation on beer and distillation columns
Working with beer and distillation columns is fundamentally different from the classical distillation process, since it becomes possible to control the amount of phlegm returned to the column with the help of a reflux condenser within a very wide range. The process is based on heat and mass transfer. In order to increase the efficiency of the process, a packing is poured into the column, which significantly increases the area of interaction between steam and reflux.
The process of partial condensation, in which wild phlegm is formed, becomes an undesirable phenomenon that worsens the accuracy of controlling the reflux ratio and separation into fractions along the height of the column. Therefore, they try to minimize partial condensation by insulating the cube and column.
The behavior of impurities during rectification is subject to their rectification coefficients, but the technology has features, the main of which is multiple evaporation and condensation of steam on the way from the cube to the refrigerator.
Each such reevaporation occurs in a certain section along the height of the column, called the theoretical plate. In the first 20-30 cm of the packed part of the column, due to repeated re-evaporation, the vapor is strengthened to a value above 90%. In this case, the impurities that fly out of the cube as part of the steam, during the passage of each subsequent theoretical plate, will change their Kp in accordance with the strength of the phlegm or steam in which they are located.
Therefore, fusel oils, which have Kp greater than one at the column inlet, acquire Kp less than one as they move up the column, and they re-evaporate in smaller quantities, and at a certain stage they completely stop. The accumulation of fusel oils occurs in that part of the column where their Kp=1. Alcohol does not allow fusel oil above, for which it is a “tail” at this strength, and below fusel oils show head properties, and when overevaporated, they rise again higher. Approximately so behave all intermediate impurity.
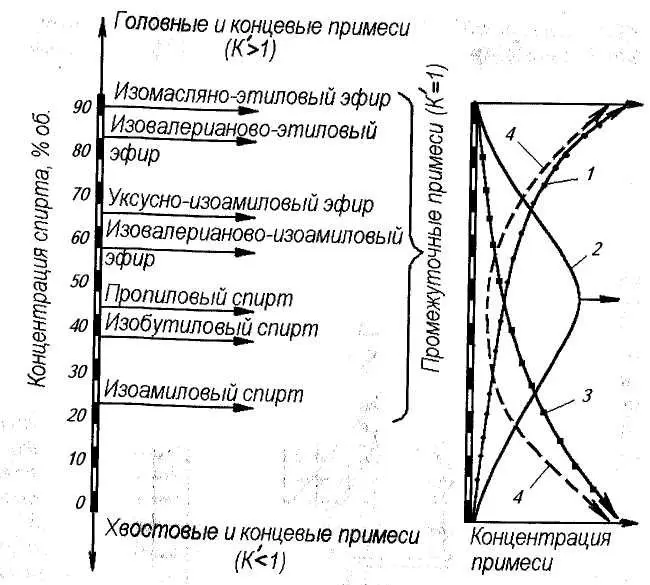
Head impurities, as they move up the column, fall into more and more strengthened steam, as a result of their Kp increases. This allows the head impurities to get into the selection zone with acceleration.
Tail impurities are exactly the opposite, having got into the column, with each new theoretical plate they sharply decrease their Kp and quite quickly, together with phlegm, find themselves at the bottom of the column, where they accumulate.
End impurities behave similarly: at a low strength, their Kp
The control of the column comes down to a simple rule: you cannot take a fraction at a rate exceeding the rate of its entry into the column. Methods for determining the moment when this speed begins to be exceeded are varied. The main thing is to understand as early as possible that the balance has been disturbed, and, by reducing the selection rate, to restore it.
In the simplest version, control is possible by two thermometers:
- distillation, showing the moment of boiling of raw alcohol in the cube, transition to the selection of “tails” and the end of the process;
- thermometer, located 20 cm from the bottom of the nozzle. In this zone, all transient processes are completed, the temperature is more or less stable and reflects the processes occurring in the column with the maximum lead in relation to the selection zone. An increase in temperature even by 0,1 degrees indicates that too much alcohol is being withdrawn – more than it enters the column, so you need to reduce the selection rate. If you do not reduce the selection, the separation into fractions in the column will worsen, and the impurities from the equilibrium position established for them will move up the column, closer to the selection.
During rectification, due to forced reflux and precise control of the reflux ratio, the most volatile fractions are obtained at the outlet, which can be taken sequentially. In addition, proper control of the column allows you to stop the movement of unnecessary impurities in it into the selection zone, accumulate them up to a certain time in the column, or even return them to the cube.
A distillation column is not so much an accurate, but rather a powerful tool for the total purification of alcohol from impurities. To obtain noble distillates, it is hardly applicable, since it requires special technologies and methods. The grouping of impurities according to volatility and the high concentration of alcohol in the column create azeotropes from them indiscriminately into necessary and unnecessary, it will no longer be possible to separate them.
When obtaining noble distillates, the goal is not to completely purify alcohol from all impurities, but to reduce their concentration in a balanced manner with partial removal of some of the most unnecessary. Requires a device with partial condensation, working on which the distiller separates the distillate into parts, and then collects a masterpiece from this mosaic.
With all the external difference, the most important properties of impurities – their volatility and the associated rectification coefficients – lie at the heart of distillation and rectification control. By controlling the phlegm number in a very limited (during distillation) or, conversely, very wide (during rectification) ranges, you can get a very different product: from a distillate balanced in terms of impurities to pure alcohol. The main thing is to understand the principles of management and use the appropriate tool in each case.
PS On March 30.03.2018, XNUMX, the article was supplemented and significantly revised, comments before this date have lost their relevance.









