In line with its mission, the Editorial Board of MedTvoiLokony makes every effort to provide reliable medical content supported by the latest scientific knowledge. The additional flag “Checked Content” indicates that the article has been reviewed by or written directly by a physician. This two-step verification: a medical journalist and a doctor allows us to provide the highest quality content in line with current medical knowledge.
Our commitment in this area has been appreciated, among others, by by the Association of Journalists for Health, which awarded the Editorial Board of MedTvoiLokony with the honorary title of the Great Educator.
Rotarix Rota, INN-vaccine is an anti-infectious (attenuated) rotavirus. Actively immunizes infants from the age of 6 weeks to prevent gastric ailments and enteritis caused by rotavirus infection. It is administered orally as prescribed by a physician. Remember that vaccination doses and dates are consistent with the standard calendar intended for small patients. It is essential to read the package leaflet before using the vaccine.
Rotarix – indications
Children become infected easily with rotaviruses, most often by hand-to-mouth transmission. The effects of such an infection are severe diarrhea in young children and infants. Their course is sometimes accompanied by vomiting and dehydration. Rotavirus infection kills hundreds of thousands of young patients every year, especially in developing countries without advanced medical care or hygiene education.
People who are vaccinated by boosting the immune system naturally produce antibodies against the types of rotavirus most common in the population. Infection of such organisms occurs more slowly or is completely stopped.
Rotarix – storage
The vaccine should be stored in a dry, dark place out of the reach of children. The package contains a glass vial, therefore special care should be taken. Attention! Rotarix should be refrigerated at 2 to 8 degrees Celsius. The formulation should not be frozen or administered beyond the expiration date, or subjected to temperatures outside the suggested range.
Rotarix – dose and composition
Rotarix contains 1,5 ml of oral rotavirus vaccine powder for suspension. The pack contains a glass vial with the contents, a pre-filled syringe filled with a solvent, i.e. a cloudy liquid with a whitish precipitate. The vaccine powder is white. An adapter is included in the package to transfer the solvent directly into the vaccine vial. After mixing, the patient should be given the suspension immediately, and if it is not consumed within 24 hours, it becomes obsolete and should be discarded immediately.
There are no restrictions on your child’s consumption of food or liquids after or before vaccination. There are no contraindications to the use of Rotarix during pregnancy or breastfeeding. Tell your doctor if you have an intolerance to sugars and have problems related to metabolism, as it may happen that the effect of the vaccine is affected by an incorrect response of the body. This is particularly important due to the area of rotavirus action against which Rotarix protects – the work of the intestines and stomach is determined by the body’s metabolic responses.
Disposal of vials and pre-filled syringes is subject to the rules of drug disposal. Do not dispose of them into sewage system or standard household or municipal bins. Doing so exposes the environment to contamination not only with vaccine residues, but also with packaging materials intended for decomposition and recycling.
The ingredients in Rotarix are: human rotavirus RIX4414 strain (ateunated), sucrose, dextran, sorbitol, amino acids, Dulbecco’s Modified Eagle Medium, calcium carbonate, xanthan gum, sterile water.
Packages containing 1, 5, 10 or 25 vaccine in the kits described above are available for sale.
The vaccination schedule consists of two doses: the first is administered from 6 weeks of age, the second preferably before the age of 16 weeks, at the latest until the end of 24 weeks. The interval between doses is at least 4 weeks.
Rotarix – side effects
Rotarix should not be used in patients who develop symptoms of hypersensitivity to the active ingredients. The following may then occur: rash, shortness of breath, swelling of the face and tongue.
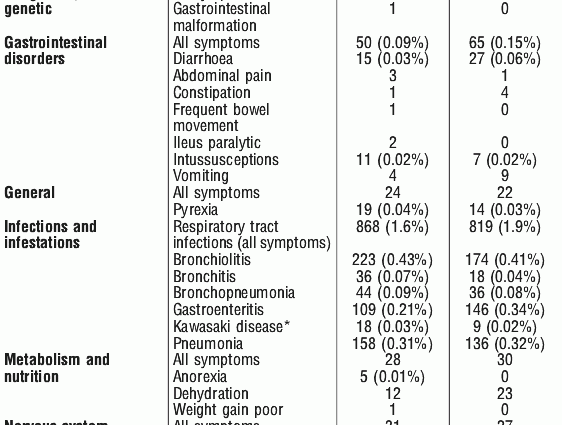
After vaccination with Rotarix, you may notice loss of appetite, irritability, vomiting or diarrhea, heavy pouring, fever and gas. These are defense effects of the organism acquiring immunity against the harmless rotaviruses contained in the vaccine. There are also symptoms of sleep disorders, constipation, upper respiratory tract infections, rash, dermatitis, muscle cramps, drowsiness, crying, runny nose.
Tell your doctor immediately if you have had side effects after a previous administration of Rotarix or another vaccine, or if you have gastrointestinal disturbances. The preparation should not be taken less than two weeks after vaccination against poliomyelitis.
People who come into contact with a baby for 7 days after vaccination should wash their hands carefully after changing nappies. It is not recommended to contact the patient with other children during this period, because transmission of rotavirus may cause erroneous immune responses of other people and, as a result, symptoms of infection.