The bacterium Helicobacter pylori inhibits the expression of a gene called RUNX3 in the stomach cells, an important transcription factor that protects against the development of gastric cancer, US scientists report in the Oncogene magazine.
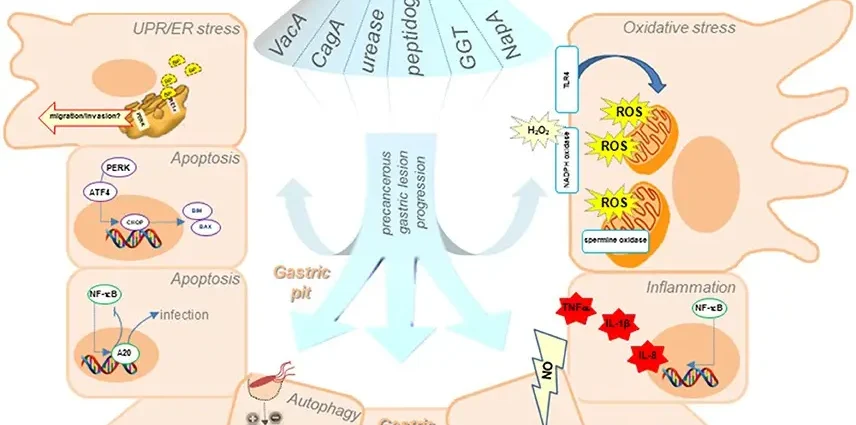
According to statistics, two thirds of people in the world live with Helicobacter pylori (H. pylori) infection, a bacterium that can survive in the hostile environment of the stomach. Most H. pylori carriers never develop the disease, but sometimes the bacteria lead to disease – inflammation, ulcers, and in some cases stomach cancer – the second most lethal cancer in the world.
H. pylori known to cause disease has a gene in its genetic material that encodes a protein called CagA. Bacteria introduce this protein into the cells lining the stomach walls, which causes numerous disruptions to normal cell signaling and their function.
It is known from other studies that a transcription factor called RUNX3 is an important gene that inhibits the growth of gastric tumors – loss of expression of this gene usually results in the development of cancer. RUNX3’s job is to sustain the production of factors that degrade damaged stomach cells. Until now, however, it was not known why the activity of this protective protein in stomach cells is sometimes inhibited.
Professor Lin-Feng Chen of the University of Illinois and his team studied H. pylori-infected gastric epithelial cells to see if the bacterium had an effect on RUNX3 expression. It turned out that the infection of cells with a H. pylori strain carrying the dangerous CagA gene led to a significant decrease in the level of the RUNX3 protein in the cells. Helicobakter pylori lacking the CagA gene do not affect the level and activity of RUNX3 in any way.
Subsequent studies of human gastric epithelial cells showed that the proteins CagA and RUNX3 physically interact with each other through special domains, which causes the RUNX3 protein to be destroyed by cellular removal of unnecessary molecules, the so-called ubiquitination.
Now Professor Chen’s group is investigating the molecular mechanism by which CagA steers RUNX3 down the path of degradation. Scientists hope that a molecule can be developed that will prevent the interaction of the two proteins and the degradation of RUNX3. In the future, such a drug could be used to prevent stomach diseases caused by H. pylori. (PAP)