Contents
In 2014, Home Distiller forum user Gabriel 61 published his algorithm for obtaining distillate from grain raw materials. In a few years, the method gained popularity among moonshiners and became known as the “Gabriel method” or “Gabriel distillation”. However, due to the large volume of information that is difficult to perceive and the insufficient structure of the material, not all distillers correctly understood the essence of the technique – they began to use it with critical errors or for unsuitable raw materials. In this article I will try to explain all the important points.
Theory
To improve the quality of the drink, the mash is distilled at least twice and divided into fractions: “heads”, “body” and “tails”. Detailed description of the factions at the link.
Due to the lower boiling point than ethyl alcohol (78,37 °C), the “heads” evaporate earlier, so they can be selected before ethyl alcohol. The situation is similar with “tails”, only their boiling point is higher than that of ethyl alcohol, so the concentration increases when almost all of the ethyl alcohol (“body”) has already been collected.
However, there are so-called “transitional fractions”, which in theory should behave like “tails”, but in practice can come out together with the “heads” and the “body”. The problem is complicated by the fact that these “adapters” are considered one of the most harmful.
During the distillation of grain raw materials, almost all “transitional fractions” contain up to 90% isoamyl alcohol (isoamylol or “izika” in the jargon of moonshiners), which is more harmful than methanol, therefore they fight with “izik” in the first place.
In its pure form, isoamyl alcohol boils at a temperature of 130 ° C, that is, it is a classic “tail”, however, when mixed with water and ethyl alcohol, the temperature drops significantly. The lower the concentration of ethyl alcohol in the cube, the more actively isoamylol evaporates and enters the “body” selection.
The process of boiling isoamyl alcohol in Braga begins when the cubic concentration of ethyl alcohol falls below 60% vol. This means that even after three or more distillations of moonshine with strengthening, only “heads” and “tails” can be separated, but the concentration of isoamyl alcohol, on the contrary, may increase.
The content of impurities in relation to ethyl alcohol in the distillation process is called the rectification coefficient. For the most common impurities in mash, the rectification coefficient is shown in the graph.
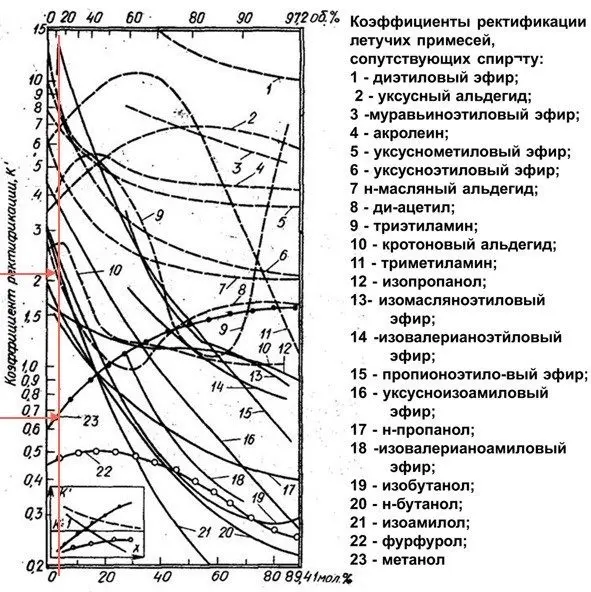
The graph shows the relationship between the selection of ethanol and isoamylol.
| The concentration of ethyl alcohol in the cube (%) | The amount of ethanol in the selection (parts) | The amount of isoamylol in the selection (parts) |
| 10 | 1 | 2-3 |
| 20 | 1 | 2 |
| 40 | 1 | 1 |
| 60 | 1 | 0,2 |
If the cubic concentration of ethyl alcohol is 60% or more, then the minimum amount of isoamyl alcohol is included in the selection. It is on this regularity that the entire process of “Gabrielian” distillation is built.
The essence of the Gabriel method
First, the mash is distilled at maximum speed to almost zero ethyl alcohol content in the cube. In this case, the output is immediately divided into the First and Second body – T1 and T2.
The first body (T1) contains 50% of absolute alcohol. With this approach, up to 70% of isoamylol and other harmful “transitional fractions” accumulate in this fraction.
The second body (T2) has a lower strength (ethanol concentration) and almost does not contain isoamyl alcohol, but it is rich in “tail impurities”, which largely form the taste of grain distillates – in a certain amount (but not very large) their presence in the finished drink is desirable.
Then each “body” is worked on separately. T1 is distilled on a column with strengthening due to the formation of reflux in order to increase the concentration of alcohol in the VAT residue. Moreover, the greater the strengthening (separation), the better. “Heads” and “tails” are selected as usual. Rectification is the best option, and one distillation in a conventional distiller practically does not purify the selection from isoamylol, therefore it is ineffective.
To clean T1 from isoamyl alcohol on a classic moonshine without strengthening, you will have to do several distillations without diluting with water. It is necessary that in the end the distilled liquid has a strength of 70-80% vol. Working with such a strong cubic bulk is not safe! In addition to the easy ignition of concentrated alcohol, the moonshiner is waiting for another problem – the minimum amount of liquid in the cube, here you need to use cubes of the minimum volume.
They work with T2, as with ordinary grain moonshine: they distill with or without fortification (at the discretion of the distiller), select “heads” and tails.
As a result, T1 and T2 are mixed in the proportions chosen by the moonshiner. Then they are sent to a barrel or, after a few days of rest, grain moonshine is drunk “white”.
Practical example of Gabriel distillation
It is easier to understand how everything works on a specific example. Suppose you want to distill 20 liters of mash with a strength of 10% vol. according to Gabriel.
For the first distillation (obtaining raw alcohol), you need to have a distiller with a minimum length of the steam pipeline, and ideally also with an insulated cube and tubes. A short steam line and insulation minimize the amount of wild phlegm – condensate returning to the cube, in which there is a certain proportion of isoamylol. That is, you need to add as little as possible strengthening of raw alcohol. For the second distillation T1 and T2, on the contrary, it is desirable to have a column with reinforcement.
algorithm
- Calculate the amount of absolute alcohol (AC), for this, multiply the volume of mash in liters by its strength in percent and divide by 100 (20 * 10 / 100) = 2 liters.
- Pour the prepared mash into the distillation cube. Distill at maximum speed on a simple short and preferably insulated distiller. 1% of the initial output of the volume of mash (20 ml) is collected separately and poured out, these are “heads”.
- Collect T1 in an amount of 50% of the AC: (2 * 0,5) u1d 52 l. It is absolute alcohol, and not just a distillate! To do this, you need to periodically control the strength of the output. For example, if the strength of the liquid in a full liter jar is 520% by volume, then it contains 480 ml of absolute alcohol and another XNUMX ml of AC must be collected.
- Collect T2. To do this, select the distillate until the strength in the stream drops below 0,5% vol. or up to a temperature in the cube of 99-100 ° C. The liquid will turn cloudy with a specific smell, this is normal.
- Overtake T1 on equipment with maximum strengthening – mash, and even better distillation column with the selection of “heads” and “tails”. You will get two outputs: almost concentrated isoamylol and purified alcohol. It is clear that it is the second faction that will be considered further T1, sometimes it is called T1.2. You can also use a conventional distiller, but then you will first have to bring the strength of the bulk to 70-80 rpm by several distillations.
- Distillate T2 like regular grain moonshine, adjusting the amount of “tails” at your discretion to leave grain notes, but not make the drink too fusel. For example, up to a fortress of 40% vol. in a stream.
- Mix T1 (T1.2) and T2 in any proportions. Pour the finished blend into barrels for aging or dilute it to a drinkable strength, and after a short rest, you can proceed to the tasting.

Limitations and disadvantages of the Gabriel method
The main disadvantage of the Gabriel method is its limited application. As the author of Gabriel 61 himself wrote, his distillation method is suitable only for grain (and not malt) distillates, and during the development the task was to create an optimal balance between fusel oil and grain aroma.
Even when working with quality malts and pure malt mashes, Gabriel 61 prefers conventional distillation to its method. This means that the Gabriel method can only be useful for cheap grains and/or low quality malts.
In the case of fruit and sugar brews, the Gabriel method is useless. The fact is that the aroma of fruit and berry distillates is formed by esters – the head fraction. If you select a distillate according to Gabriel, then the unique fruity notes will disappear. True, to solve this problem, “fromGabrielization” was invented, we will consider this modification further. And in sugar mash, in principle, there is no aroma or taste that needs to be preserved.
Disadvantages of Gabriel distillation:
- in its classic form, it is not suitable for sugar and fruit brews;
- requires a variety of equipment – from a distiller and an insulated cube to a column with reinforcement;
- laborious – you need to separate the distillate, do more distillations, then blend;
- complex – requires experience in distillation for competent separation of “heads” from “tails” and creating a blend.
From Gabrieling fruit and sugar brews
The method from Gabrielivation was published on the Grain Drinks forum by user alexeyT in 2016. The bottom line is that by three or four successive distillations, raw fruit alcohol is strengthened up to 86% vol. on a conventional moonshine still (preferably copper, according to the cube-refrigerator scheme, without a steamer), each time with a small selection of “heads”.
Algorithm of actions for 20 l of mash (10% vol.)
- Make the first distillation, select 10-50 ml of “heads”, finish the selection when the strength falls below 10% vol in the stream.
- Make 2-3 more distillations without diluting the resulting yield until the final strength of the product is 84-90% vol. Each time, collect 10-50 ml of “heads” and finish the distillation at a strength in the jet below 10% vol.
- In the final distillation of a very strong fruit distillate, select the “heads” until the unpleasant odor disappears. It is important not to overdo it, otherwise the aroma of the drink will be weak. Finish the selection when the smell of fuselage appears in the jet (usually corresponds to a temperature in the cube of 80-81 ° C.
During all distillations, you need to be extremely careful and remember that it is dangerous to work with such a strong liquid in a cube!









