Contents

Among the many elements involved in the metabolic processes in the human body, none is as important as iron. The highest concentration of iron in erythrocytes – red blood cells, to be more precise – in hemoglobin. A small proportion of iron is found in the blood plasma and in the tissues of the human body as part of ferritin, hemosiderin and transferrin.
If the body loses iron for any reason, iron deficiency anemia occurs. To detect it, the determination of serum iron in the blood is carried out.
The rate of iron in the body
Iron is found in the blood serum in connection with transferrin (25%), its binding and transporting protein. To prescribe an analysis for the determination of serum iron, a base is required – a low level of hemoglobin, usually found as part of a complete blood count.
During the day, the concentration of Fe in the body fluctuates:
14,3-25,1 mmol / l in men;
10,7-21,5 mmol / l – in women.
Differences are due to the presence of menstrual bleeding in women, with age they are erased. Iron deficiency is increasingly defined with age in both women and men.
The norm of a trace element depending on gender and age:
Gender and Age | The norm in μmol/l |
Infants under one year old | 7,16 – 17,9 |
Children and teenagers from 14 to XNUMX years old | 8,95 – 21,48 |
Youths and adult men | 11,64 – 30,43 |
Girls and adult women | 8,95 – 30,43 |
To get objective indicators, you need to follow the rules when passing the analysis:
Donate blood on an empty stomach (at least 12 hours before analysis);
At least a week in advance, stop taking tablets for the treatment of IDA;
Do not take the test a few days before the blood transfusion.
To determine the level of Fe, blood serum is used, placing it after sampling in a dry test tube, never used before, not exposed to detergents.
Functions and biological significance of Fe in the blood
No living organism can do without iron, because it performs the following functions:
Provides tissue respiration with the help of a microelement concentrate in the blood;
Provides full activity of skeletal muscles.
Erythrocytes and blood hemoglobin bind oxygen that has entered the lungs from the environment, carry it throughout the human body, and carry out carbon dioxide formed as a result of gas exchange.
The divalent ion Fe plays a leading role in the activity of hemoglobin to ensure respiratory activity. Under the influence of strong oxidizing agents, it becomes trivalent, turning into methemoglobin. Red blood cells containing methemoglobin pass through hemolysis and are destroyed. As a result, tissues enter a state of acute hypoxia.
The synthesis of iron by the human body is impossible, it comes with food: meat, fish, vegetables and fruits. For the full extraction of Fe from food, a large amount of ascorbic acid is needed, then the absorption of the trace element from animal products increases several times.
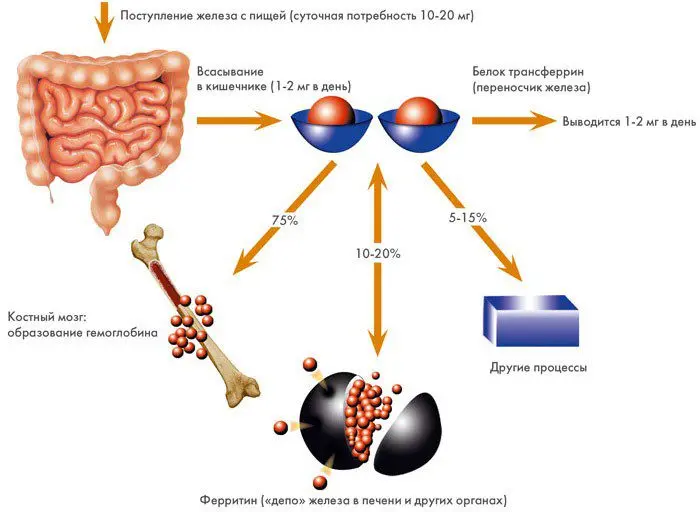
There is a precise mechanism for regulating the absorption of iron in the small intestine: if it is not enough, enhanced absorption of the microelement begins, if there is an excess of iron, the blocking of the process begins. Iron absorption does not occur in the large intestine. The need for Fe to enter the body is 2-2,5 mg, women need more, since they monthly lose at least 10 mg of this trace element with every 20 ml of blood.
Increased content
An excessive concentration of Fe in the blood, as well as its lack, speaks of existing pathologies. Since the mechanism for regulating the absorption of this trace element is quite reliable, an increased content of iron indicates its breakdown or an increased breakdown of red blood cells and the release of ions of this element corresponding to it.
Causes of elevated Fe levels in the body:
Hemolytic, folic acid deficiency, aplastic, B12– anemia, thalassemia;
Increased absorption in the small intestine in violation of restrictions (hemochromatosis);
Hemosiderosis due to an overdose of drugs with a high content of Fe, or numerous blood transfusions;
Lead poisoning, side effects of oral contraceptives, sideroahrestic anemia, which caused a failure of hematopoiesis in the bone marrow;
Hepatitis of any etiology, liver necrosis, hepatopathy, cholecystitis in acute form.
When examining test results, it is important to take into account the possible use of oral iron preparations by patients.
Lack of iron in the body

Since iron is not produced by the human body, and few people calculate its content in the diet, after a while its deficiency begins to be felt. Fe deficiency causes anemia and is manifested by the following symptoms:
“Flies before the eyes”;
Dry and pale skin;
Dizziness;
Brittle nails;
Dryness and hair loss.
The reasons for the decrease in the concentration of iron in the circulatory system:
Lack of an element in food when switching to a vegetarian diet, excessive consumption of fatty foods that do not contain iron, or dairy diets when calcium does not allow Fe to be absorbed.
Increased need for various trace elements, including iron, during lactation and pregnancy, during the period of rapid growth in childhood and adolescence.
Diseases of the gastrointestinal tract leading to iron deficiency anemia, as they prevent the absorption of iron through the intestinal walls: gastritis, enterocolitis, enteritis, condition after resection of the stomach or small intestine, neoplasms.
Osteomyelitis, myocardial infarction, rheumatism, purulent, inflammatory, septic infections, tumors with rapid growth dynamics, when Fe is actively absorbed from blood plasma by phagocytes.
Hemosiderosis, when hemosiderin accumulates in the tissues of the body, which leads to a decrease in the level of iron in the blood.
Chronic renal failure leading to deficiency of erythropoietin in the kidneys.
Nephrotic syndrome, in which iron is actively excreted in the urine.
Prolonged bleeding: menstrual, hemorrhoidal, epistaxis, etc.
Active reaction of hematopoiesis with a pronounced use of iron.
Malignant and benign tumors, cirrhosis, liver cancer.
Obstructive jaundice due to stagnation of bile (cholestasis).
Deficiency of vitamin C, which promotes the absorption of iron.
How to increase the level of iron in the blood?
It is difficult to raise the level of Fe without knowing the reasons for its decrease. Even a diet rich in trace elements will not be able to influence this process if the absorption of iron is impaired. In order not only to ensure the transit of iron through the gastrointestinal tract, but to fully influence the cause of its decrease, you need to be guided by the doctor’s recommendations and undergo a full examination.
Products to saturate the diet with iron:
Meat: rabbit, steam lamb, veal, beef, turkey, goose. Pork fat does not have Fe at all in its composition.
The liver of animals, which is the organ of hematopoiesis. It is important to be aware of the possible high levels of toxins, and not include offal in the diet too often.
Bird eggs contain little iron, but they are rich in phospholipids, vitamins B1, B12;
Buckwheat as the best product against iron deficiency anemia.
The use of vegetables and fruits to replenish ascorbic acid, which helps to absorb iron through the intestinal wall. Citrus fruits (lemon, orange) and sauerkraut are best suited for this. You can eat vegetables and fruits rich in Fe: apples, peas, beans, prunes, spinach, but of these, the microelement penetrates the gastrointestinal tract in a minimal amount.

Foods rich in calcium (cottage cheese, milk, cheese) interfere with the full absorption of Fe, so they are consumed separately from foods rich in this trace element.
Video story about iron and iron deficiency anemia:









