Contents
- Is there a difference between ethyl alcohol and ethanol
- Types of ethanol used in food production
- Where is ethanol used, in addition to the production of alcohol
- Can it be used in pure form?
- Optimal and maximum dose
- Why is methyl alcohol so terrible and how to distinguish it from ethyl
- Methods of obtaining
- What products contain, in addition to alcohol
- Chemical characterization of ethanol
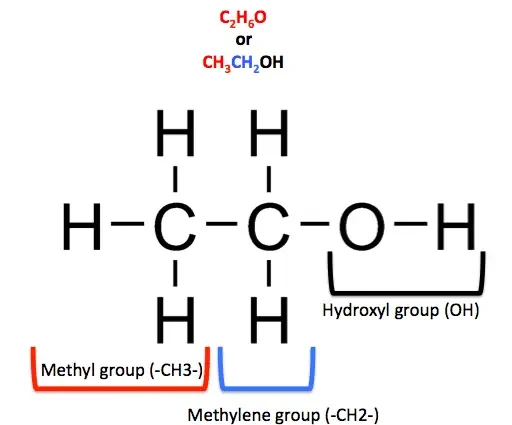
Everyone knows that strong alcohol contains ethyl alcohol. Rummaging in the back of memory, some will even remember the formula of a high-grade substance: С₂H₅OH, (СH₃СH₂OH). This is where the knowledge of most of us about ethyl alcohol ends.
For the most curious, Vzboltay has collected interesting facts and useful information about the main alcoholic ingredient, as well as answers to popular questions.
cocktails with vodka
Is there a difference between ethyl alcohol and ethanol
No. In fact, these are 2 synonyms that refer to the same substance. Although ethanol is often used as a more general name, uniting under its banners both ethyl and wine alcohol, both food and medical. But the statement “ethyl alcohol = ethanol” is true.
Types of ethanol used in food production
The alcohol obtained after purification in a special installation contains 95,6% ethanol. Depending on the presence of third-party impurities in it, several categories of substances are distinguished (given in order from higher quality to lower quality):
Alpha
Made from wheat and rye, used for the production of elite vodkas.
Luxury
Made from grain and potatoes (the latter – no more than 35%), subjected to thorough cleaning, used to obtain premium vodkas.
Extra and Basis
Made from grain and potatoes, used for alcohol of the middle price category.
Supreme purification
Obtained from a mixture of potatoes, grains, beets and molasses (in different proportions), minimally cleaned, used in the production of tinctures, liqueurs, economy-class vodkas.
1 grade
Not used in the alcohol industry.
Where is ethanol used, in addition to the production of alcohol
In medicine, ethyl alcohol is used as an outdated type of antiseptic, as a basis for making tinctures, compresses, etc.
It also found application in industry, in particular in the chemical and fuel industries. It is noteworthy that the first car, created by Henry Ford in 1880, ran on ethanol.
Today, the substance is used as fuel for rockets and internal combustion engines. Also, ethanol is a part of many cosmetics and detergents.
Can it be used in pure form?
In no case. When ingested in its pure form, ethyl alcohol causes mucosal burns and severe intoxication.
You can drink it only in diluted form and in reasonable doses.
Optimal and maximum dose
You can calculate the safe consumption rate for a particular person using a special formula: 1,5 ml of pure alcohol per 1 kg of body weight. It needs to be adjusted depending on the ethanol content in the drink.
For example, the rate of consumption of vodka, including 40% alcohol, will be 3,75 ml per 1 kg of body weight. If alcohol intake is extended in time for several hours, a slight increase in the dose is allowed, but not more than 25% (if your own health is dear to you).
A lethal single dose is 4–12 g of ethanol per 1 kg of body weight. It is curious that, contrary to popular belief, ethyl alcohol is not at all a panacea for bad mood. This effect is short-term, but in general the substance is considered a depressant that depresses the nervous system, not to mention other consequences of abuse (cirrhosis of the liver, problems with the gastrointestinal tract, various types of cancer, and much more).
Why is methyl alcohol so terrible and how to distinguish it from ethyl
All types of alcohol when consumed in their pure form are dangerous. But methanol causes the worst effects.
At best, when using it, a person can go blind, at worst, die. Moreover, methanol acts with lightning speed.
In order not to add to the list of victims of surrogate alcohol, it is better to refrain from buying it in dubious places and carefully choose alcohol.
If there is any doubt about the quality of the purchased (or donated) drink, you can check it in one of 3 ways:
set fire to
A good product containing ethanol will have a bluish flame, a bad one (with methanol) will have a green flame.
Dip a piece of fresh potato into the drink
If there is methanol in alcohol, the vegetable will turn pink.
Pour a solution of potassium permanganate into a glass
The substance will not react to ethanol in any way, and when interacting with methanol it will hiss and foam.
It is noteworthy that the antidote in case of methyl alcohol poisoning is ethyl alcohol, which is administered intravenously or ingested in a certain dosage.
Methods of obtaining
The classic way to produce ethanol is the good old fermentation. A variety of vegetable raw materials are used as raw materials: fruits with a high content of carbohydrates, potatoes, grains, rice, corn, etc.
The drink obtained as a result of fermentation contains only 15% ethanol and needs further processing (distillation, purification).
For industrial purposes, ethanol is isolated synthetically (using ethylene hydration or cellulose hydrolysis).
With the help of special reactions, it is possible to obtain absolute alcohol, which practically does not contain water.
What products contain, in addition to alcohol
In some drinks obtained as a result of fermentation: in kvass, kefir, the so-called “non-alcoholic” beer. The ethanol content in them can vary from 0.01 to 0.05%.
Chemical characterization of ethanol
Colorless, volatile
monoatomic liquid substance
lighter than water
Melting point = -114,1°C
Boiling point = 78,39 °C
Auto-ignition temperature = 400 °C
Highly flammable (at 18°C)
good solvent
Relevance: 05.07.2019
Tags: vodka, mash, moonshine