Contents
- General information: what is cancer?
- Nobel Prize in Medicine 2018: what is the essence of the discovery
- The principle of operation of the method
- What drugs are used for cancer immunotherapy?
- Method risk assessment
- CAR-T cancer gene immunotherapy method
- What is the point of this treatment?
- Scheme of CAR T-cell therapy
- Registered drugs for CAR-T
- Side effects of CAR T-therapy
- What advances have been made in CAR-T cancer gene immunotherapy?
- What else are scientists working on?
In medicine, there is a serious breakthrough in the treatment of cancer. In the last few years, the results of clinical studies have been published. All of them ended with a full-fledged victory over malignant neoplasms.
Every year in Russia, cancer is diagnosed in 600 people. And 000% of them die from the disease. In the first year after the discovery of pathology, death occurs in 50% of people. Globally, this figure is much lower.
Much is known about cancer, but at the same time very little is known. This paradox leads to the fact that people continue to die from the disease. A particular problem is the advanced forms of oncology, in which tumors give metastases. It is difficult to cope with such a pathology. The most favorable prognosis for those patients whose cancer is diagnosed in the early stages of development. Nevertheless, a breakthrough in the treatment of certain types of cancer has been significant.
General information: what is cancer?
Cancer is a malignant tumor that contains mutated cells. They quickly divide and grow, affecting nearby tissues. In a certain period, the tumor begins to spread its metastases throughout the body.
Tumor neoplasms can develop from various cells of the human body: from the dermis, bones, muscles, nerve fibers. Therefore, neoplasms grow in various parts of the body. The more doctors know about the location of the tumor and the structure of its cells, the higher the chances of successfully getting rid of the neoplasm. Specialists have the opportunity to draw up an optimal treatment regimen. Despite this, it remained a mystery why some tumors cause rapid death of the patient, others respond well to therapy, and still others appear again after a few years.
Methods of treatment of cancerous tumors that are used in practice:
Operation. It is designed to remove the primary focus of the tumor and metastases, to save the person from the complications that were caused by the growing neoplasm.
Chemotherapy. Treatment is aimed at reducing the size of the tumor and removing metastases. With its help, it is possible to reduce the risk of recurrence of pathology.
Radiation therapy. This treatment method affects the tumor at the local level, which allows you to suppress the growth of the neoplasm.
Hormonal therapy. It is indicated for patients who suffer from breast or prostate cancer.
The main disadvantage of chemotherapy and radiation therapy is that during the treatment, not only atypical, but also healthy cells suffer. Skin, mucous membranes and bone marrow are affected. It is in the latter organ that blood cells are formed. Therefore, patients undergoing chemotherapy develop a lot of side effects. They are diagnosed with anemia, they begin to suffer from intestinal problems, their hair falls out. Even with the use of the most modern drugs and techniques, doctors do not have the ability to protect healthy cells in the body.
Nobel Prize in Medicine 2018: what is the essence of the discovery
On October 1, 2018, the Nobel Prize in Medicine and Physiology was awarded in Stockholm. 2 scientists received it at once – this is the American James Ellison and the Japanese Tasuku Honjo. The award was presented for their research in the field of cancer treatment.

Immunologist James P. Allison, professor at Cancer Center and. Monroe Anderson of the University of Texas, member of the US National Academy of Sciences and the US National Academy of Medicine. The scientist is now 70 years old.

Immunologist Tasuku Honjo. He is a professor at Kyoto University, where he has been teaching since 1984. The scientist is a member of the US National Academy of Sciences, the German Academy of Naturalists “Leopoldina” and the Japanese Academy of Sciences.
The merit of scientists is to develop an innovative approach to cancer treatment. Their method differs from chemotherapy and radiation therapy used worldwide. The name of the method is Immune checkpoint therapy. This is cancer immunotherapy, which allows you to reduce the activity of atypical cells and prevent the destruction of the immune system. The use of this method causes the immune system to actively attack the neoplasm cells. [1].
Scientists were able to detect the body’s ability to suppress the activity of T-lymphocytes. These immune cells are responsible for destroying cancerous tumors. If the mechanisms of suppression of T-killers are blocked, then lymphocytes are “released” and begin to independently eliminate tumor neoplasms.
The principle of operation of the method
The human immune system is made up of many cells. If we consider it globally, then the body’s defense is represented by activators (stimulators) and inhibitors (inhibition process). When these two systems balance each other’s work, a person’s health is excellent. The immune system is able to cope with any disease on its own.
T-lymphocytes are white blood cells that are represented by suppressors, killers and helpers. Each cell type is responsible for a specific function. T-helpers must recognize their own and foreign cells. When abnormal cells are detected, they stimulate the immune system to work harder. T-killers and phagocytes begin to arrive at the problem area, and the process of antibody production is activated in parallel.
T-suppressors are responsible for the suppression of immune processes in the body. They do not allow the immune system to show excessive activity. This prevents the development of autoimmune diseases.
When a tumor begins to grow in the body, proteins are formed in it that have an atypical structure. They are different from those proteins to which the body is accustomed. T-cells react to them as if they were foreign objects.
The tumor, in an effort to maintain its own viability, is trying to deceive the immune system. Cancer cells have the ability to camouflage themselves. They remove defective proteins from their surface, or destroy them. Tumors are even capable of producing special substances that reduce the activity of the human immune system. The more active the neoplasm, the less chance the immune system has to cope with it.
Discovery of James Ellison. This scientist has found a way to unlock the immune system using antibodies to get rid of the brake protein. The doctor studied the functions of the cellular protein of T-lymphocytes (it was given the name CTLA-4). He managed to establish that it was he who was blocking the work of the T-killers. The scientist tried to find a way to unlock immunity. In the process of ongoing research, the doctor decided to create an antibody that could bind the brake protein and interfere with its work.
The experiments were carried out on rodents with cancer. The scientist was trying to figure out if blocking CTLA-4 would help activate the immune system and make it work against the tumor. [2].
Ellison’s main merit is that he was the first to put forward a version of the relatively “unhealthy” appearance of CTLA-4 on T-killers. That is, this protein is formed on immune cells so that the tumor can stop them. Each active killer T cell has an inhibitory molecule that competes with other molecules to receive a signal from the immune system (signals can be of two types: turning on and off the body’s defenses). If CTLA-4 is located on the surface of the T-killer, then it intercepts the signals coming from T-helpers and the immune system does not direct efforts to fight cancerous tumors.
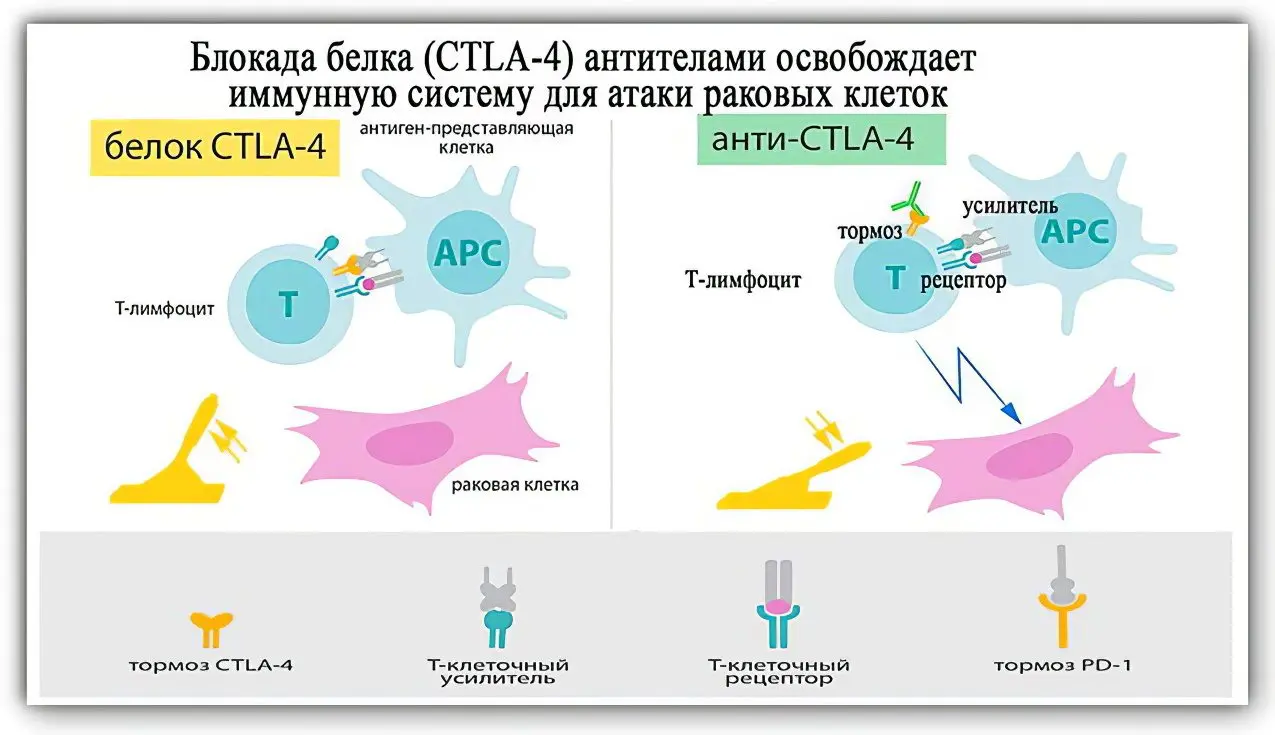
Discovery of Dr. Tasuku Honjo. In 1992, this Japanese scientist identified PD-1 protein molecules on the surface of T-lymphocytes. This abbreviation is deciphered as Programm cell death protein 1, which in translation from English means “programmed cell death protein”. The scientist found that it works like a brake. Protein not only inhibits the growth of neoplasms, but also blocks T-killers [3].
Tasuku Honjo synthesized antibodies to PD-1, which made it possible to eliminate the existing blockage and increase the activity of the immune system against cancer cells.
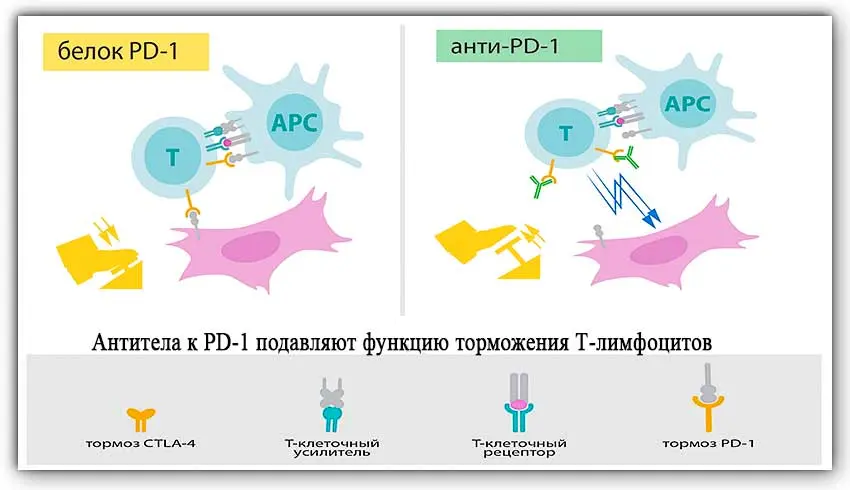
Scientists have called PD-1 and CTLA-4 and their signaling pathways immune checkpoints. They were able to show how, by destroying elements that inhibit the immune system, cancerous tumors can be dealt with.
More than 15 years have passed since the opening. During this time, preparations containing inhibitors of immune checkpoints have been developed and put into practice. In the treatment of cancer, 1 drug that blocks CTLA-4 and five drugs that block PD-1 are used. This difference in the number of drugs created is explained by the fact that many tumors also have PD-1 on their surface. Therefore, PD-1 blocking drugs allow targeted action on the tumor, while CTLA-4 blockers affect only the activity of T-killers. In addition, there are fewer complications from the use of PD-1 blockers.
What drugs are used for cancer immunotherapy?
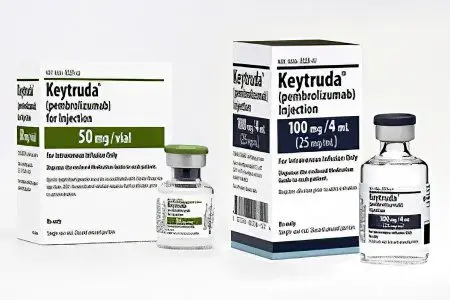
The first clinical trial of the drugs was conducted in 2006 on people with cancer. It involved a remedy called Nivolumb. This drug is a PD-1 blocker. However, it was approved for the treatment of cancer patients only in 2014. At the same time, all trials of Pembrolizumab, produced by Merck, were completed.
The following drugs have been registered in Russia:
Pembrolizumab (Keytruda). It is used to treat lung cancer and melanoma. [4]. Its undoubted advantage is its high efficiency in the treatment of metastatic malignant tumors. The price of one bottle is 3290 euros.
Opdivo (Nivolumab). This drug is an analogue of Keytruda, but it costs less. It has been successfully used to treat kidney cancer and melanoma. The cost of the drug is $915 for a 40 mg pack and $2200 for a 100 mg pack. Depending on the supplier and manufacturer of the drug, the price for it may differ.
Yervoy (ipilimumab). The drug is prescribed for adults and children over 12 years of age at a dosage of 3 mg / kg. A full treatment course will require 4 doses. Enter it within 1 hour 30 minutes. The procedure is carried out 1 time in 21 days. The cost of one bottle with a dosage of 50 mg / 10 ml: 4200-4500 euros, and with a dosage of 200 mg / 40 ml – 15 euros.
Tecentrik (atezolizumab). This drug is prescribed for the treatment of urothelial and non-small cell lung cancer. The price of the drug depends on intermediaries and the place of purchase. In the USA 1 bottle costs $6500-8000.
These drugs are used as independent units, and in various combinations. This treatment is indicated for patients with inoperable metastatic melanoma, Hodgkin’s lymphoma, recurrent and metastatic squamous cell carcinoma of the neck and head, and inoperable bladder cancer.
Russia also produces immunological preparations for the treatment of cancerous tumors. It must be understood that the practical application of checkpoint therapy has just begun. Naturally, in a few years there will be much more drugs in this group. They can be used to treat other types of cancer. The cost of therapy will be more affordable, as most of the costs are left behind.
Method risk assessment
Immunotherapy should not be taken as a panacea for cancer. The use of these drugs does not guarantee a 100% recovery of the patient. Medicines do not work on all types of cancerous tumors. What matters is the genotype of a particular patient.
Treatment with immunological drugs is associated with the risk of side effects. They mainly come down to the development of autoimmune reactions. Since the main active substances affect the human immunity, activating it, it starts to work too actively. Therefore, the patient often has autoimmune inflammation of the internal organs.
Another disadvantage of such drugs is that they can be used to treat adults. They are not prescribed for small patients.
In some patients, these drugs do not work at all, as tumor cells are especially agile and hide from attacks by an enhanced immune system.
CAR-T cancer gene immunotherapy method
CAT-T is an innovative method of cancer treatment, which was presented by the American Society of Clinical Oncology (ASCO) in the report “Advances in Clinical Oncology 2018” [5].
The therapy is based on the ability of T-lymphocytes to fight chimeric antigen receptors. In short, this treatment method is called CAR-T (chimeric antigen receptor T-cell).
In order to create chimeric CAR antigen receptors, Zelig Eshhar from the Weizmann Institute of Sciences, which is located in Rehovot, Israel, first thought. A chemist and immunologist by training, he was the first to obtain transgenic T-lymphocytes containing CAR. The discovery was made in his laboratory.
However, clinical trials of this new cancer treatment were not completed until 2017. In the course of their implementation, 2 medicines Kymriah and Yescarta were created and approved for use.
If we consider CAR-T globally, then it can be attributed to several treatment methods at once: to gene, cell and immunotherapy.
What is the point of this treatment?
CAR-technology allows you to set a new program for the patient’s immune cells outside his body. Scientists create CAR T cells that have the ability to find cancerous tumors and destroy them. The resulting CAR cells are used for adoptive immunotherapy (adoptive is one of the varieties in cancer treatment).
CAR T cells are obtained using ex vivo technology, that is, from human blood. T-lymphocytes are isolated from it, which are responsible for protecting the body from cancer and other pathological cells. The DNA encoding CAR is then inserted into the chromosome of the T cell. Due to such changes, T-lymphocytes begin to produce chimeric receptors on their surface. They allow T cells to find markers located on the surface of cancerous tumors. Once detected, the immune system sends a signal to attack. CAR T cells are propagated outside the human body, after which they are injected into the patient’s blood.
If a genetically modified cell meets a normal healthy cell, then it does not react to it. When a cancer cell is detected, the chimeric antigen receptor “sees” a marker on it, for which it was previously programmed. T-lymphocyte throws on the tumor cell and destroys it, after which it begins to actively divide. This allows you to completely get rid of cancer.
Until all tumor cells are destroyed, CAR lymphocytes will not stop working. When there are no cancer cells left in the body, most of them will die. However, a small reserve will still remain in the bone marrow. If there is a relapse of the disease, then they will again begin to divide to resist cancer.
This method is suitable for the treatment of such types of tumors as:
Aggressive B-cell lymphoma.
Acute lymphoblastic leukemia in children and adults.
B-cell large cell lymphoma. It is possible to use this method to get rid of diffuse lymphoma.
Now scientists are conducting research aimed at combating other types of tumors using the CAR method.
Scheme of CAR T-cell therapy
CART refers to an innovative cancer treatment that was developed in America. The leading oncological clinics of the world have already worked out this treatment regimen. Its implementation in practice is considered safe and reliable.
Figure – methods of cell therapy ex vivo and in vivo:
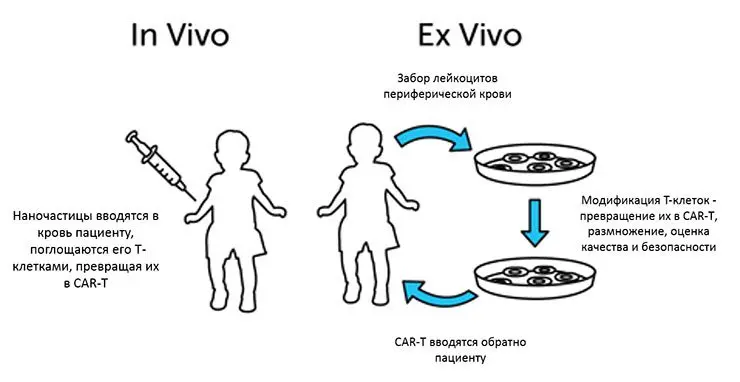
To begin with, the patient will need to undergo a series of diagnostic procedures. If there are no contraindications to CART, then the patient is prescribed treatment. It lasts for several weeks. During this period, the person will be either in the hospital or at home.
1.First step: blood sampling. Doctors use special equipment to take the patient’s blood. It is divided by isolating leukocytes. This procedure is called leukapheresis. Donating blood takes about 5 hours.
2.Second phase: processing of T-lymphocytes. Blood cells are genetically modified in the laboratory. Scientists induce the expression of chimeric antigen receptors that will seek out and eliminate tumor cells. At this time, a person may be outside the hospital walls.
3.The third stage: conducting chemotherapy before the implementation of CART. The person will need to be re-tested before the treated T-lymphocytes are administered. Sometimes it happens that further treatment with this method is no longer possible. If nothing has changed, then the patient is prescribed chemotherapy for a short period. During this period, tests will need to be taken every day.
4.Fourth stage: introduction of T-lymphocytes. Their infusion takes about half an hour, although sometimes the procedure can take up to 1 hour and 30 minutes. Then for about 5-6 hours the person should remain under medical supervision. If there is a risk of side effects, then the patient is left in the hospital for several days.
The FDA requires that CART patients be followed up for at least 15 years.
Registered drugs for CAR-T

In 2017, 2 drugs were approved that are suitable for CART. They were accepted by the US Food and Drug Administration (FDA) based on clinical trials.
Kymriah (tisagen lekleikel / tisagenlecleucel). This is the first drug produced by Novartis. It began to be massively used from 30.08.2017/25/XNUMX. Treatment can be given to children and adults under XNUMX years of age diagnosed with end-stage blood cancer [6].
The high cost does not allow the introduction of Kymriah for mass use. It will cost $475 to create gene T cells and inject them into a patient. The cost does not include hospital fees.
Although the drug is already available for use, scientists continue to study its properties. Currently, the drug is at the stage of post-marketing observational studies.
Yescarta (axicabtag ciloleukel / axicabtagene ciloeucel).This is the second drug that can be used to implement CAR T-cell therapy. It began to be used from 18.10.2017/XNUMX/XNUMX. It is produced by Kite Pharma Inc.
Treatment with this drug is carried out in patients with B-cell large cell lymphoma in adults, provided that the disease does not respond to other types of therapy and recurs. The only contraindication is the primary lesion of lymphoma of the brain or spinal cord [7].
The price of this drug is extremely high and amounts to $373. Drug manufacturers are actively looking for ways to reduce the cost of the process of its creation. This will make the drug available to more people.
Side effects of CAR T-therapy
The implementation of the CAR T-therapy method allows the cells of the immune system to detect the tumor and destroy it. However, the activation of immunity cannot pass without a trace for the body. Patients often develop severe side effects.
In order to be able to conduct CAR T-therapy, medical institutions must have a special certificate. Doctors are obliged to inform the patient about the health consequences that arise after the treatment. It is important to evaluate all possible risks.
Side effects develop on days 1-22 after the introduction of altered cells.
These include:
Weakening of the immune system, a sharp decrease in the level of leukocytes in the blood, the development of infections.
Anemia, hypotension.
Acute renal failure. This complication is rare.
Nervous system disorders. Sometimes cerebral edema may develop.
The most common adverse reaction is the so-called cytokine storm. It develops in 75% of patients. Cytokines are proteins that control the functioning of the immune system. After the meeting of the changed T-cells with the tumor, a huge amount of cytokines is released into the blood. Such a reaction is accompanied by an increase in body temperature, vomiting, diarrhea, increased weakness. If this condition is not managed for a long time, the likelihood of death increases.
In order to prevent the development of a cytokine storm, the patient is prescribed the drug Actemra (Tocilizumab) or classic NSAIDs, such as Diclofenac.
What advances have been made in CAR-T cancer gene immunotherapy?

On November 30, the results of the annual implementation of gene therapy in practice were summed up. The customer was the US Food and Drug Administration. It was this organization that gave permission for the use of medicines. The results were published in the New England Journal of Medicine. 93 patients were treated [8].
It was found that 37 patients managed to completely get rid of the disease. Another 11 people began to feel much better, but it was not possible to achieve a complete victory over cancer. Therefore, scientists concluded that the technique works by 50%.
The situation in Russia
In Russia, for the first time, CART technology was implemented in the Center for Pediatric Hematology named after N.N. Dmitry Rogachev (NMIC DGOI). The head of the long-term work is Doctor of Medical Sciences Mikhail Maschan. At the initial stage, the project was supported by the Podari Zhizn Foundation. The opportunity to implement the method within the walls of this medical institution appeared thanks to donations from the top management of Rosneft and the Doctors, Innovations, Science for Children Foundation.
In 2018, 20 children and young people with acute lymphoblastic leukemia and B-cell lymphomas received treatment. Other therapeutic methods did not allow recovery. The only hope left was CART.
What else are scientists working on?

Tremendous advances were made in the treatment of cancer in 2018. More breakthroughs are expected in 2019.
Cancer immunotherapy
In addition to the CFR T-cell therapy described, tumor-infiltrating lymphocyte (TIL) therapy is under development. This method has already made it possible to get rid of metastatic breast cancer in a 49-year-old patient. However, large clinical trials have not yet been conducted. [9].
Liquid Biopsy: An Accurate and Easy Cancer Test
A liquid biopsy will allow cancer to be diagnosed from a blood test. New tests make it possible to monitor the treatment process and anticipate a possible relapse.
Recently, there have been many tests from various companies that assure the effectiveness of their products. However, in 2018, the American Society of Clinical Oncology (ASCO) stated that most of these products cannot be used for disease detection and monitoring. This is due to the lack of proven efficacy of these tests. [10].
Reduced side effects of treatment
If in the past decades the main efforts were made to find effective ways to fight cancer, then in 2018 studies were conducted aimed at reducing the side effects of the treatment. First of all, this concerns male infertility and impaired puberty in girls after chemotherapy. Sufficient attention was paid to the prevention of cosmetic defects in appearance that occur after removal of the mammary gland, etc.
Oncological diseases and microflora of the body
Scientific articles have appeared that indicate that the microflora is able to predict the body’s response to chemotherapy [11].
Publication in the journal Nature Communications [12] indicates that certain bacteria present in the human microbiome can affect the state of the immune system and cause the growth of multiple melanoma (blood cancer that cannot be cured). It is possible that the destruction of the detected bacteria will have an impact on cancer treatment.
Organelles
Organoids are miniature organs artificially grown in a laboratory from a person’s own cells that can transform oncology. Information about this appeared in the media back in 2017. On organoids, it is possible to carry out tests of various drugs and to foresee what kind of reaction the patient’s body will give to the treatment.
These technologies have been adopted by many large organizations. Organoids are supplied to various laboratories, thanks to which it has already been possible to increase the efficiency of ongoing work on the screening of anticancer drugs.
Organoids are not an ideal medium for drug testing. These mini-organs are not supplied with blood and have no connection with other body systems. However, scientists continue to improve organelles and methods for growing them. In the future, they will be used much more actively.
Video: Professor Daniel Chen (USA) at the conference “Immuno-Oncology” (April 6, 2018, Moscow) “Cancer Immunotherapy: from theoretical foundations to breakthroughs in treatment”:









