Contents
Hydrogen indicator, which determines the acidity of the blood, or pH, or a marker of acid-base balance, is a constant value..
A shift in indicators towards the alkaline side (alkalosis), or towards acidity (acidosis) are symptoms of trouble that require urgent treatment.
If the pH index drops below 7 units, or rises above 7,8 units, a person is in a borderline state between life and death, where 6,8 on the one hand, and 8,0 units from the opposite segment of the range mean the death of the organism.
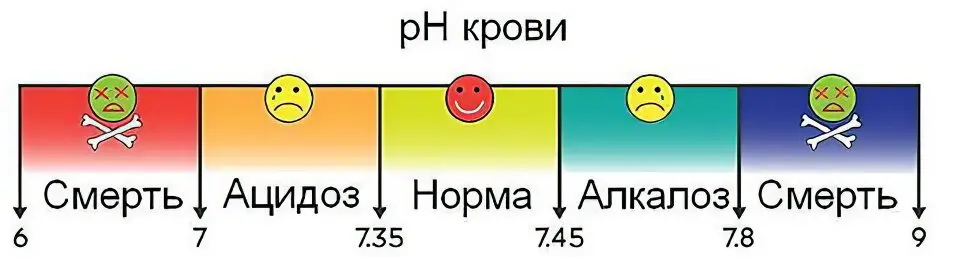
Acid-base balance: what is it?
It would seem that the entry into the digestive tract, and further into the blood, of products with an acidic or alkaline reaction should change the composition of the blood. In fact, buffer systems of the body ensure the stability of the acid-base balance , not allowing fluctuations outside the safe range.
List of buffer systems:
Bicarbonate (hydrocarbonate) system – provides at least 50% of the adaptive abilities of the hemostasis system;
Hemoglobin system – 35% safety;
Blood protein system – 10% buffer capacity;
Phosphate system – 5-6% buffer safety.
These systems, supporting the vital activity of the body, prevent the shift of the acid-base balance in any direction, despite the fact that the body consumes products of various composition. Buffer systems have an inexhaustible margin of safety, since they are constantly supported by the excretory system, which is activated at the level of reflexes when it is necessary to remove metabolic products.
How systems work
Basic hydrocarbon system
The hydrocarbon system includes two components: H2CO3 and NaHCO3. Between them and the acids and alkalis entering the blood, chemical reactions constantly occur.
Strong alkali reaction:
NaOH + H2CO3 → NaHCO3 + H2O
The sodium bicarbonate formed as a result of this interaction is soon excreted by the urinary system.
Neutralization of the incoming acid occurs as follows:
HCl + NaHCO3 → NaCl + H2CO3
As a result of the reaction, carbon dioxide is formed, which is excreted by the lungs into the environment.
The bicarbonate buffer system is the most sensitive to changes in pH and therefore responds to them immediately.
Hemoglobin, protein and phosphate systems of blood
Blood hemoglobin, with the help of a red pigment, reacts to changes in acidity by binding oxygen, or giving it to the surrounding tissues. The acidity of the red pigment of hemoglobin changes by 0,15 units, acting as a neutral salt or as a weak acid, depending on the circumstances.
The reaction of hemoglobin when an alkaline base enters the blood:
NaOH + HHb → NaHb + H2O
The interaction of hemoglobin when acid enters the blood:
HCl + NaHb → NaCl + HHb
The protein buffer system is involved in maintaining the pH balance depending on the concentration and structure of protein compounds.
The phosphate buffer safety system maintains the acid-base balance in the urine, in the interstitial fluid, and in the cytoplasm of the cell.
pH of different human blood systems
The acid-base index of arterial blood saturated with oxygen is 0,01-0,02 units higher than the same index of venous blood containing carbon dioxide in excess.
The acidity of blood plasma, which has a balance of hydrogen ions and hydroxide ions, corresponds to the acidity of the blood as a whole.
The pH of other media (serum) may have a small range of values. Blood plasma withdrawn from the hemostasis system is devoid of fibrinogen. Its acidity is of practical importance when plasma is used for blood typing using hemagglutinating sera.
Acidosis and alkalosis of the blood
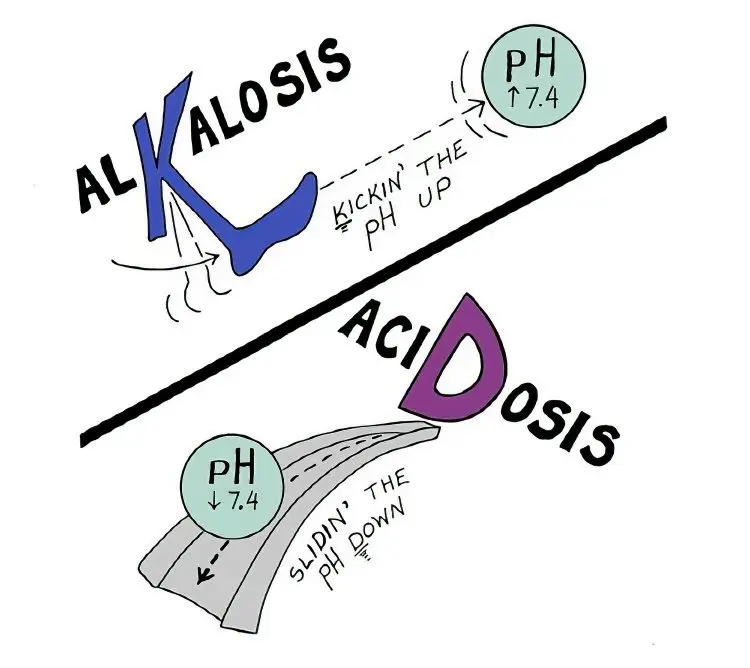
The shift of the hydrogen balance to the acidic or alkaline side can be compensated and uncompensated. It is determined by the alkaline reserve – the volume of carbon dioxide displaced by a strong acid from 100 ml of plasma. The norm of this indicator is 50-70 ml of CO2.
CO2 below 45 ml – uncompensated acidosis;
CO2above 70 ml – alkalosis.
Types of alkalosis:
Gas – occurs with altitude sickness, with hyperventilation of the lungs, provoked by an increased return of carbon dioxide to the lungs, goes into hypocapnia;
non-gas – distinguish between alimentary alkalosis, which comes with food, and metabolic alkalosis, associated with changes in metabolism.
Types of acidosis:
Gas – triggered by delayed CO release2 lungs, goes into hypercapnia;
Non-gas (alimentary) – occurs with the accumulation of metabolic products, when they enter from the gastrointestinal tract;
Primary renal – occurs when there is a violation of resorption in the renal tubules, accompanied by a loss of alkalis.
With a significant deviation of the hydrogen index from the norm, qualified medical care is required. When he stays in the extreme values of the range, with satisfactory health, it is important for the patient himself to pay attention to the state of his health.
The main causes of pH disturbance are the use of “harmful” foods, alcohol, and smoking. If the patient does not have information, he will not pay attention to his health until he is in a state of acute pathology.
You can normalize the acid-base balance with the help of dietary nutrition, but when the old lifestyle returns, the pH values uXNUMXbuXNUMXbwill return to their previous values.
To maintain the indicator within the normal range, it is necessary to follow the rules of a healthy diet, regimen, and the implementation of recreational activities.









